MAT-2105201 v1.0
XIENCE 28 showed that XIENCE™ Stent with 1-month DAPT had no increase in ischaemic events and a significantly lower rate of severe bleeding (BARC 3-5) compared with 6-month DAPT. These data were used to support the CE Mark for a 1-month DAPT indication in High Bleeding Risk Patients.
XIENCE™ STENT is proven safe with 1-month and 3-month DAPT in HBR patients.1
1-Month DAPT Indication for High Bleeding Risk Patients
XIENCE 28 vs XIENCE 90 - First Look

1-Month DAPT Indication for High Bleeding Risk Patients

XIENCE 28 vs XIENCE 90 - First Look
XIENCE 28 Results1
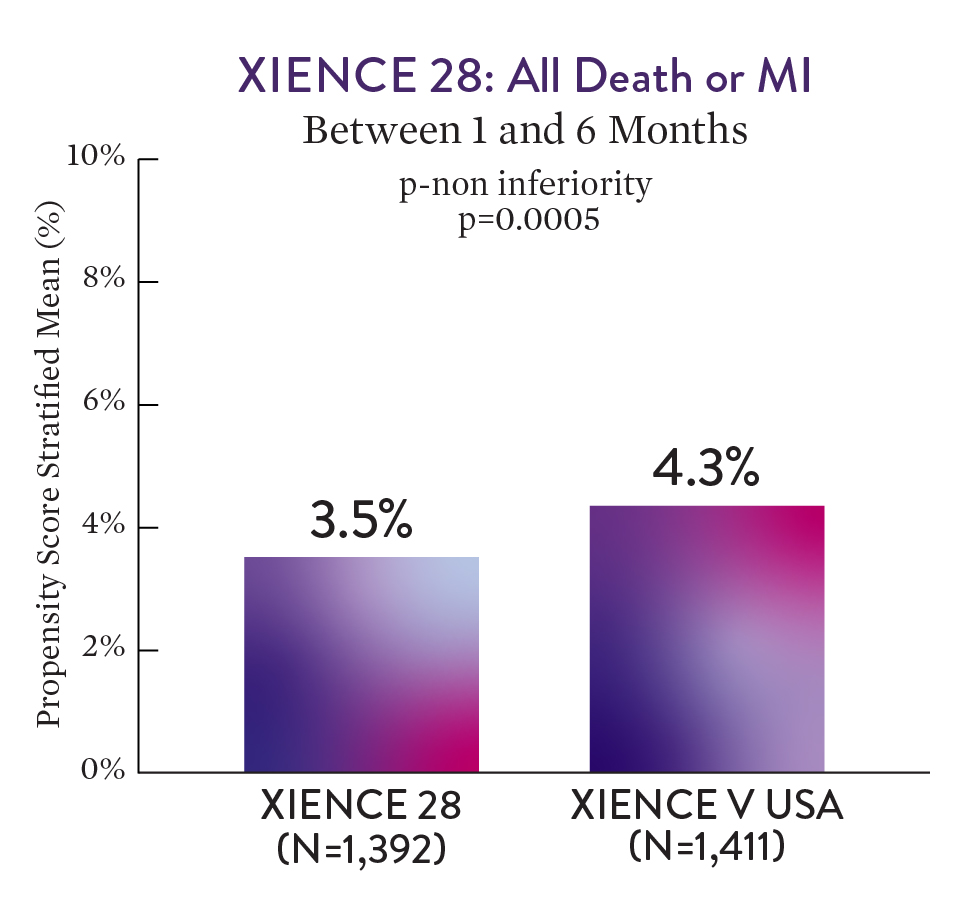
XIENCE™ Stent with 1-month DAPT showed no increase in ischaemic events versus 6-month DAPT — all death or MI.1
XIENCE 28 met its primary non-inferiority endpoint and included over 1600 high-bleeding risk patients.1
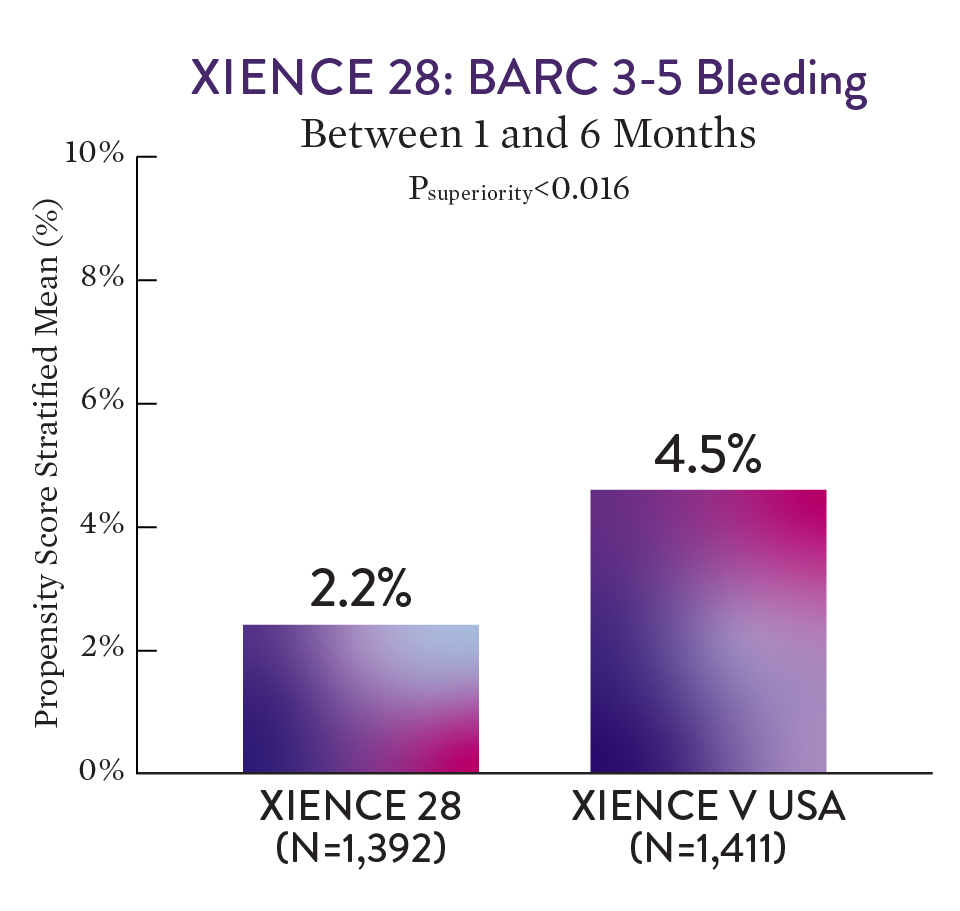
XIENCE™ Stent with 1-month DAPT had a significantly lower rate of severe bleeding versus 6-month DAPT (BARC 3-5).1
For BARC 2-5 — XIENCE™ Stent with 1-month DAPT showed numerically lower bleeding rate versus 6-month DAPT.1
Note: PS stratified analysis for BARC 3-5 bleeding was not pre-specified.
Note: BARC 2-5 was a powered secondary endpoint.
XIENCE™ Stent shows low ST rate and is significantly more thromboresistant than other DES.1,2
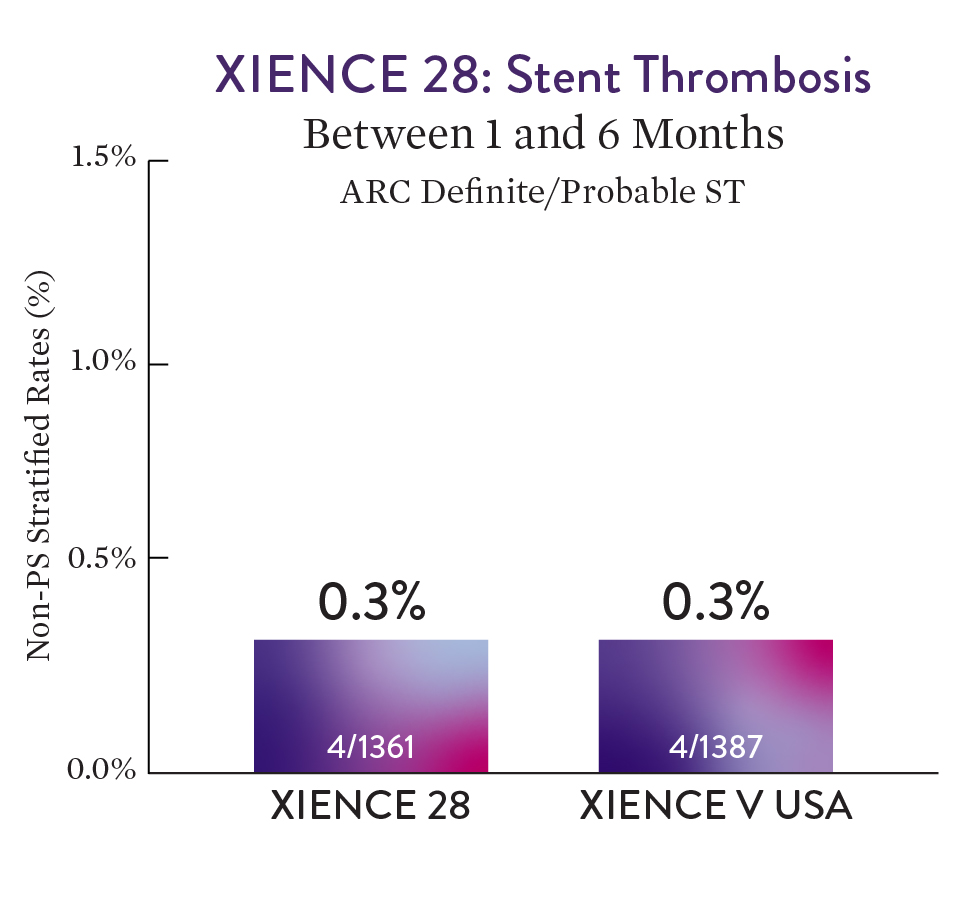
XIENCE™ Stent with 1-month DAPT showed no increase in ST versus 6-month DAPT.1
XIENCE™ Stent with 1-month DAPT had a low rate of Definite/Probable stent thrombosis of 0.3% for the 1-month DAPT group.1
XIENCE™ Stent is significantly more thromboresistant than other DES.2
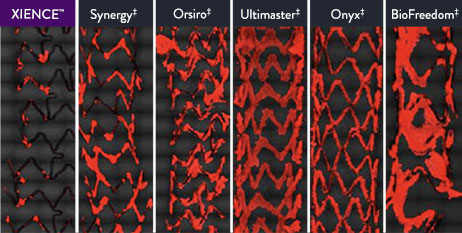
XIENCE™ Stent's fluoropolymer is significantly more thromboresistant than other DES2
XIENCE™ Stent shows significantly (p<0.01) less platelet adhesion vs. other DES.2
XIENCE 90 Results1
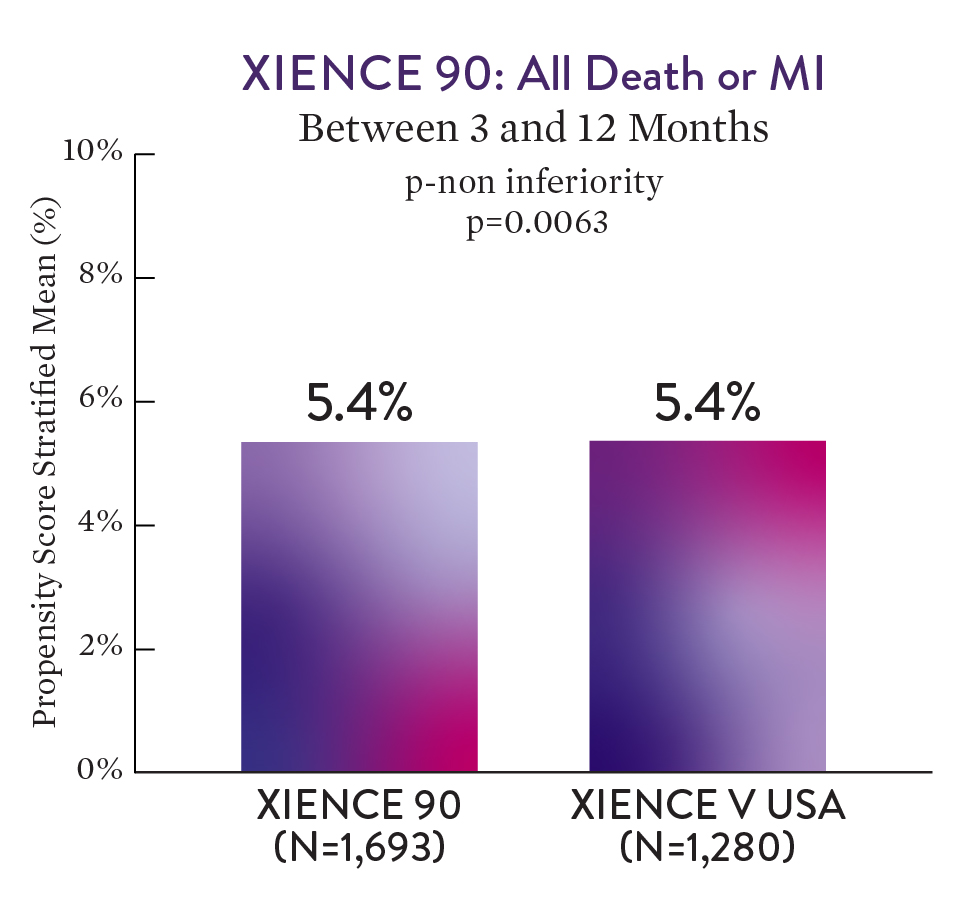
XIENCE™ Stent with 3-month DAPT showed no increase in ischaemic events versus 12-month DAPT — all death or MI.1
XIENCE 90 met its primary non-inferiority endpoint and included over 2000 high-bleeding risk patients.1
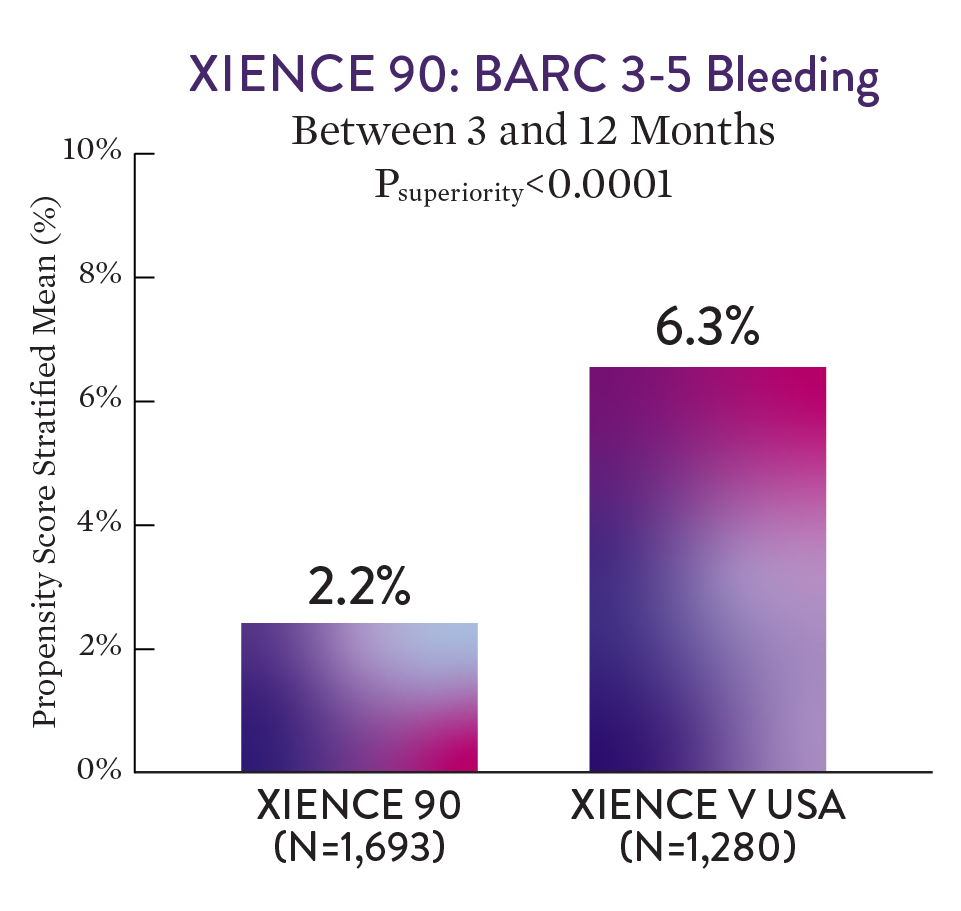
XIENCE™ Stent with 3-month DAPT had a significantly lower rate of severe bleeding versus 12-month DAPT (BARC 3-5).1
For BARC 2-5 - XIENCE with 3-month DAPT showed numerically lower bleeding rate versus 12-month DAPT.1
Note: PS stratified analysis for BARC 3-5 bleeding was not pre-specified.
Note: BARC 2-5 was a powered secondary endpoint.
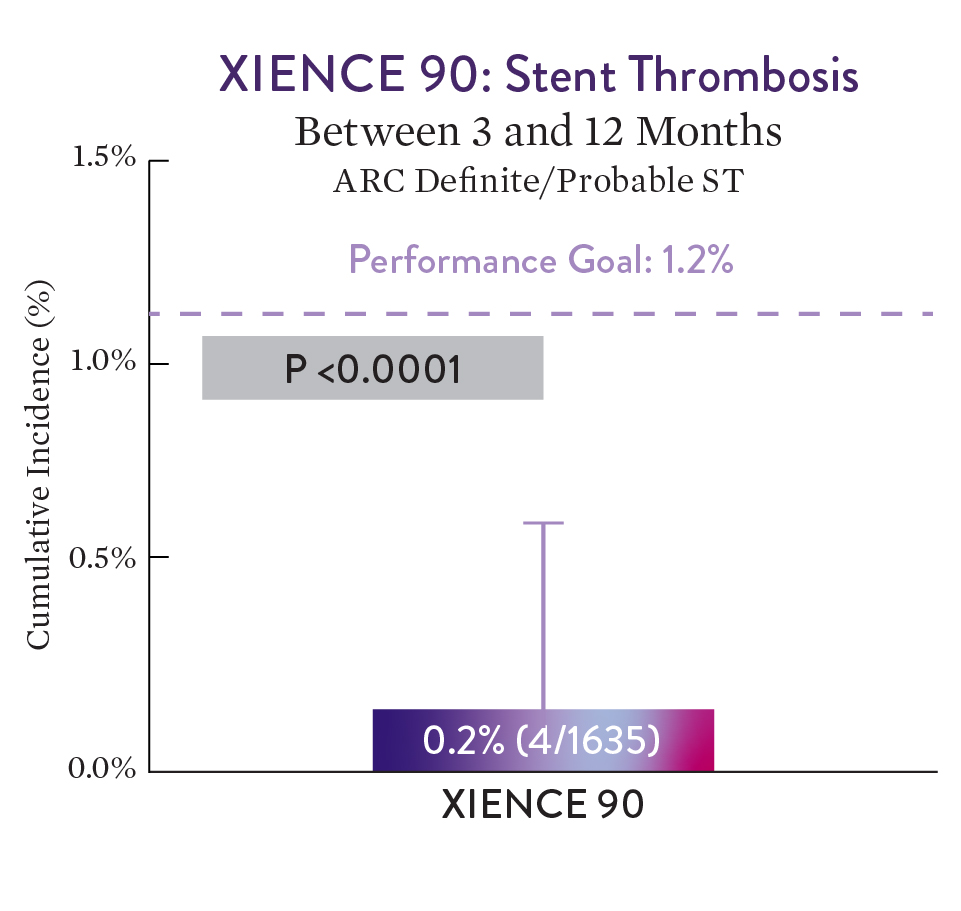
XIENCE™ Stent with 3-month DAPT met its performance goal for ST.1
XIENCE 90 met its performance goal for ST and showed a low rate of Definite/Probable stent thrombosis of 0.2% for the 3-month DAPT group.1
References
- XIENCE Skypoint - Instructions for Use. Mehran R, et al. J Am Coll Cardio Intv. 2021;14:1870-1883.
- Sato Y, et al. Int J Cardiol. 2021;338:42-49.
MAT-2500436 V1.0

