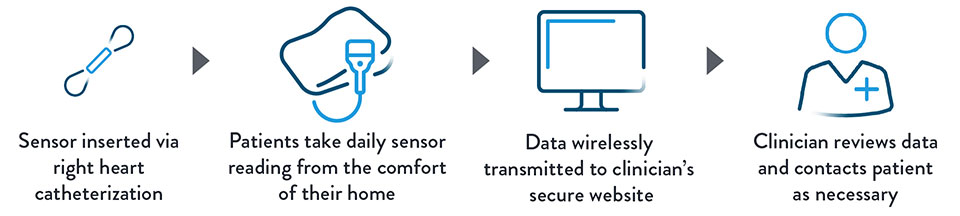
The CardioMEMS™ HF System provides pulmonary artery (PA) pressure remote monitoring using a small sensor. Permanently implanted in the distal pulmonary artery via a safe right heart catheterization procedure, the sensor measures changes in pulmonary artery pressure. These changes are a surrogate measure for fluid retention in the lungs caused by worsening heart failure.
Patient-initiated sensor readings are wirelessly transmitted to a secure website for clinicians to access and review. Directly monitoring PA pressure not only alerts you if a patient’s heart failure is worsening, it also allows you to intervene earlier, adjusting medication or making other treatment changes, often before a patient experiences any symptoms.
The Merlin.net Patient Care Network (PCN) is a remote monitoring platform that presents pulmonary artery (PA) pressure data as actionable information. This information can guide earlier, more appropriate intervention for each patient.
The platform incorporates remote PA pressure data with Implanted Electronic Device (IED) diagnostics, such as:
MAT-2116106 v3.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0