Perclose™ Devices are the only vascular closure
devices approved to close after all
Pulsed Field Ablation (PFA) Systems §,1,†


ENABLE immediate and durable hemostasis1 with >96% freedom from major access site related complications at 30 days2,3,4,5
ENHANCE your patient's comfort and satisfaction over manual compression1,5,17
EMPOWER higher EP lab efficiency with ambulation in as early as 2 hours1,2,4* and same day discharge2,3,4,5**
Learn more about how you can finish your procedure with confidence.

The Perclose™ ProStyle™ closure device achieves rapid hemostasis of femoral access sites by approximating the edges of the vessel wall with a surgical suture. The benefits of suture-mediated repair include promoting primary intention healing with less scarring18 and decreased time to hemostasis, ambulation, and patient discharge.4,19
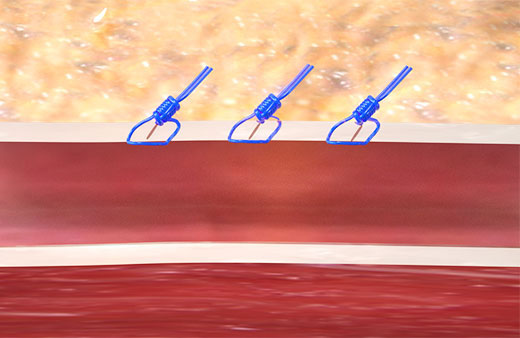
Unlike collagen based VCDs,† Perclose™ Prostyle™ has no re-access restrictions.1
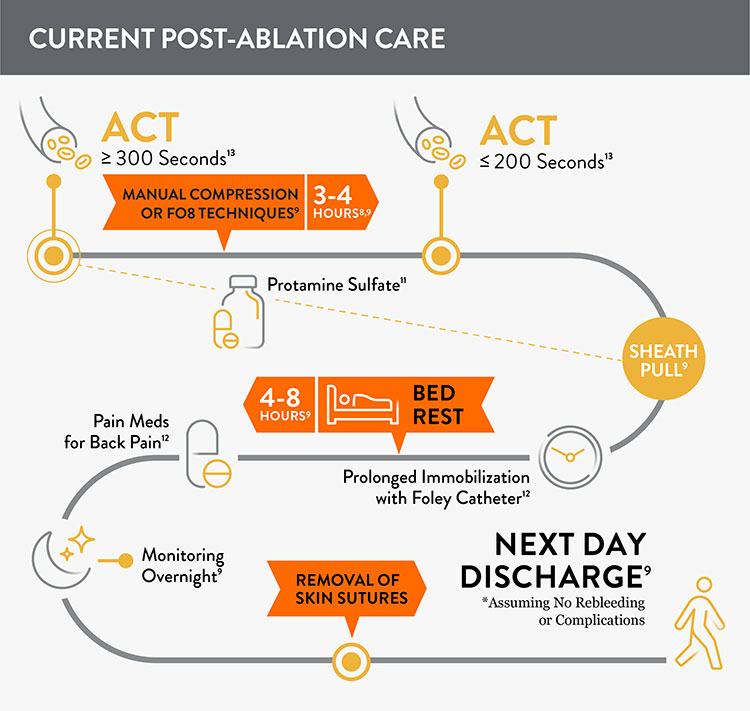
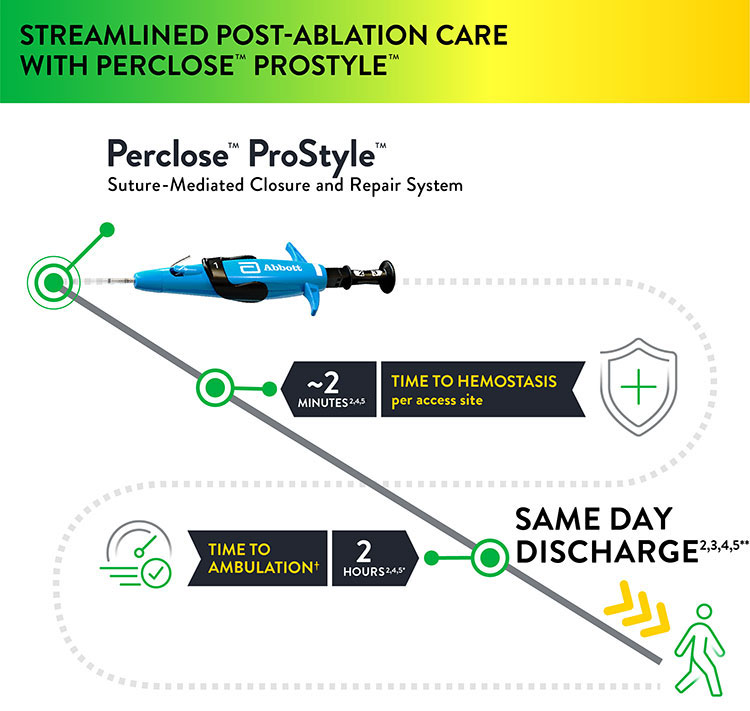
Prior to adopting Perclose™ ProStyle™ SMCR System, EPs completing an atrial fibrillation ablation or other procedures may find that patients require:
The Perclose™ ProStyle™ closure device transforms an otherwise lengthy patient recovery to a shorter recovery time, which in turn leads to a positive patient experience:
The STYLE-AF (randomized two-arm study, n=125) compared Perclose™ Systems to Figure-of-Eight sutures after PFA, and Cryo ablations for Afib. The Perclose™ Study group demonstrated a significantly reduced hemostasis, ambulation, and discharge eligibility times and increased patient satisfaction with their bedrest time relative to the Fo8 control group.26

1,000+ patients have demonstrated the safety and effectiveness of Perclose™ ProStyle™ in closing multiple CFV access sites across multiple real-world investigator sponsored studies (ISS).2,3,4,5,24,25,26
The use of Perclose™ ProStyle™ Suture-Mediated Closure and Repair System can help:
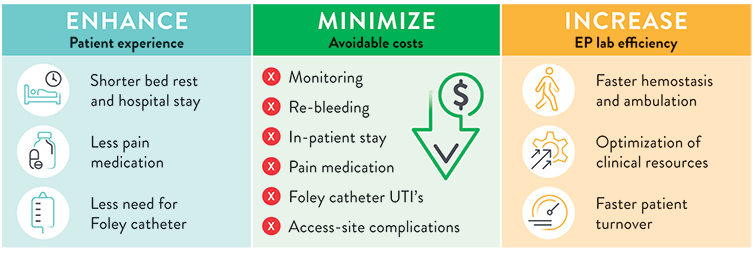
SOURCE: S. Verma. Adopting a Strategy of Early Ambulation and Same-Day Discharge for Atrial Fibrillation Ablation Cases - EP Lab Digest - May 2019.

Each of these brief videos offers details of multiple device deployment using Perclose™ closure devices.
AFib RF Ablation with
4x Venous Access Sites
Brett Gidney, MD
Santa Barbara, U.S.
AFib RF Ablation with
4x Venous Access Sites
Hemanth Ramanna, MD
The Hague, The Netherlands
2x Large-Bore Vascular Closure
Magnus Settergren, MD
Solna, Sweden
More case video recordings are available, with physicians offering additional details about their use of the Perclose ProGlide™ device.
Yes, Perclose™ ProStyle™ can be used in conjunction with a variety of AFib ablation techniques, including Pulsed Field Ablation (PFA) for venous sheaths up to 24F ID (29 OD).1†
At the time of this publication, only Perclose™ Devices can close after all PFA systems including Farapulse‡ (13F Inner Diameter (ID), 16.8F Outer Diameter (OD)). §,1,†
Using a VCD has several advantages given the following factors during EP procedures such as AF ablations:
All of these issues are mitigated when using the Perclose™ ProStyle™ closure device.
Yes, Same-Day Discharge has been shown to be safe, and it is being used to reduce the total cost of care and to enhance the patient experience. The use of vessel closure devices makes it possible for hospitals to implement Same-Day Discharge.4,17,20,21
With the Perclose™ ProStyle™ device you can achieve and confirm complete hemostasis on the table with a suture-mediated repair of the access site. Other advantages of the Perclose ProGlide™ SMC System include:
Find out more about primary healing with the Perclose ProStyle™ vessel closure device.
No, there is only one Perclose™ ProStyle™ SMCR System. Multiple Perclose™ ProStyle™ devices can be used, if necessary, for large-bore vascular closure.
Because this device achieves immediate and durable hemostasis, patients may sit up immediately in bed.1 Clinical evidence for cardiac arrhythmia treatments with multiple access sites has shown that patients safely ambulated within 2 hours2,4,5,* and were eligible for same-day discharge after successfully closing with Perclose™ devices.2,3,4,5**
It achieves hemostasis by approximating the edges of the vessel wall with a surgical suture, allowing primary intention healing to begin. Primary intention healing minimizes scarring and allows for immediate reaccess if needed. View primary intention healing images with vessel closure device.18
Contact your local Abbott representative for a training opportunity.
The "Pre-Close" Technique involves the Perclose™ ProStyle™ suture being placed around the access site before the index procedure, and it is required before using sheath sizes > 8F.1
See Deployment and Instructions for Use for additional information.
Visit the official Perclose™ ProStyle™ website for more information on the features, deployment, clinical data, Perclose™ ProStyle™ videos, and ordering information related to Perclose™ ProStyle™ SMCR System.
§ At the time of this publication (Oct 2024), for the following PFA systems: Farapulse‡# (13F Inner Diameter (ID), 16.8F Outer Diameter (OD)), PulseSelect‡ (9.5F ID; 14F OD), and Varipulse‡ (8.5F ID; 11.5 OD)
†As compared to Angio-Seal‡, ExoSeal‡, Celt ACD‡, MANTA‡, Mynx‡, Vascade‡. Data on file at Abbott.
*As observed in the VACCAR trial (≥1.4 hours) and the PRO-PVI trial (≥1:26 time to ambulation) after successful close with Perclose device(s) in patients who have undergone cardiac arrhythmia treatments with multiple common femoral venous access sites.
**As observed in the PROFA trial (80% discharged within 3:34h) and the PRO-PVI trial (≥3:38 hours post-procedural time to discharge) after successful close with Perclose device(s) in patients who have undergone cardiac arrhythmia treatments with multiple common femoral venous access sites.
MAT-2002002 v8.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0