MAT-2403640 v2.0 | Item approved for U.S. use only. ©2025 Abbott. All Rights Reserved.
Assert-IQ™ Insertable Cardiac Monitor (ICM) delivers meaningful value through efficient, accurate, and continuous patient monitoring.
It features:
This next-gen ICM provides you with reliable data to enable better care decisions for your patients.4
Assert-IQ ICM is a Bluetooth®-enabled ICM that provides reliable, long-lasting cardiac monitoring with no compromises in features.
6+ years* may be preferred for longer term monitoring, such as those undergoing therapy and at risk of developing further arrhythmias such as AF.
3+ years* may be preferred for more traditional reasons for monitoring, such as diagnosing syncope, palpitations, or detection of atrial fibrillation (AF) within cryptogenic stroke patients.
The Assert-IQ 3 ICM offers another 3-year battery life option that encapsulates only the essential and most valued ICM features.


Extend your patient monitoring beyond diagnosis and catheter ablation. Long-term monitoring with an ICM helps detect AF recurrence, even if asymptomatic. 7-11

Timing of AF recurrences after the initial catheter ablation for n=2,194 patients that underwent catheter ablation for AF between 2004 and 2019.6
Assert-IQ EL+ is the only ICM that can provide 6+ year monitoring for AF management.*,4,12-18
Designed to provide industry-leading accuracy for arrhythmia detection, Assert-IQ ICM features new algorithm enhancements that have been tested and proved to reduce false detections.1
Enhanced algorithms reduce false detections by 98.7% for AF and Pause while maintaining 97.7% of true EGMs.1
New 5-step AF detection discriminator
for R-R interval patterns and P-waves in EGMs to verify if an event is true or false.
Intelligent diagnostics provide trends and timelines of clinically relevant information to provide a holistic view for patient management and empower data-driven decisions.
Premature
Ventricular
Contractions
(PVCs)
Elevated Heart Rate with and without Activity
Body Position &
Posture Changes
DirectTrend™ Viewer


Assert-IQ ICM offers simple and secure remote programming, allowing you to control any connected device without requiring your patients to physically visit the clinic.*** This can reduce clinic workflow burden and ensure transmitted data is unique to the patient.
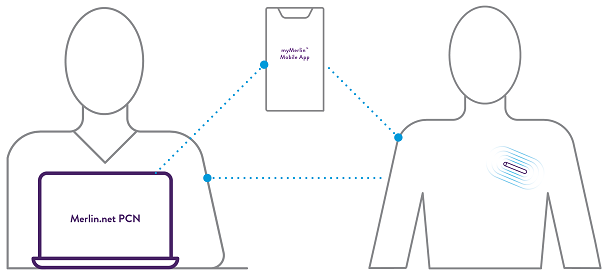
All parameters can be remotely programmed by the clinician.^
Explore a three-part allied health professional series, led by a top expert in Merlin.net PCN and ICM patient management.
†As of 12.31.23. Reveal LINQ‡ User Manual, LINQ II‡ User Manual, Lux Dx‡ User Manual, Biomonitor III‡ User Manual, Biomonitor IIIm‡ User Manual, Biomonitor IV‡ User Manual.
*Data on File, Abbott - Report 90984075.
**Key Episodes is a feature of Merlin.net™ Patient Care Network.
***Available on DM5300/DM5500.
^Not applicable for Name, DOB, and reason for monitoring
Additional references
MAT-2303940 v10.0
Stay Connected