The Perclose™ ProStyle™ Suture-Mediated Closure and Repair (SMCR) System enables an improved post-cardiac ablation experience:
PercloseTM ProStyleTM has the broadest indication* for venous and arterial sheaths.
In addition, this vessel closure device also has a proven and trusted track record from more than 20 million repairs.3
Learn how you can finish your procedure with confidence with the Perclose™ ProStyle™ SMCR System.

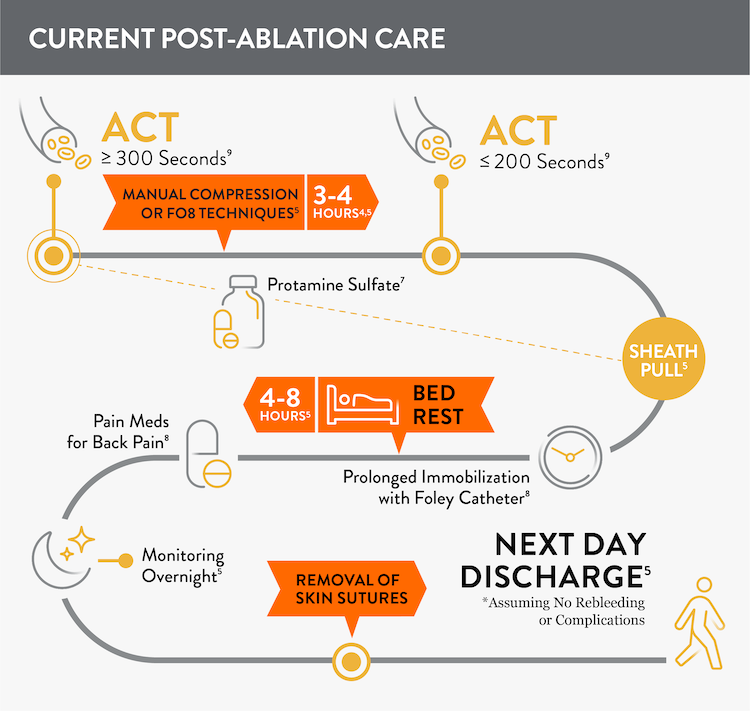
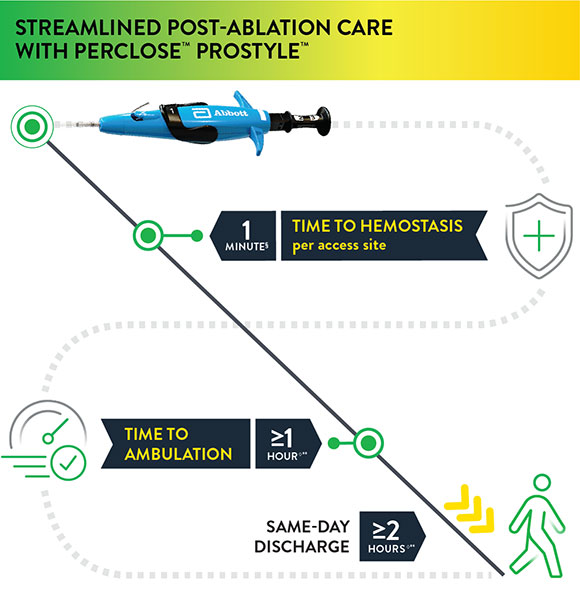
Prior to adopting Perclose™ ProStyle™ SMCR System, atrial fibrillation, including pulsed field ablation or other procedures may find that patients require:
The Perclose™ ProStyle™ closure device transforms an otherwise lengthy patient recovery to a shorter recovery time, which in turn leads to a positive patient experience:
§observed median time to hemostasis per access site (DUS) IDE Trial
◊As per the IFU, patients who have undergone cardiac arrhythmia treatments with multiple access sites in a single femoral vein of one or both limbs may be ambulated one hour or more and may be eligible for same-day discharge two hours or more after successful closures with Perclose™ devices based on the judgment of the physician.
The rapid time to hemostasis allows EPs to verify the status of the closure while the patient is still under their care1, enhancing confidence in the entire procedure from access to closure. Moreover, it’s potentially beneficial to the EP lab and hospital staff when patients are quickly ambulating, freeing up beds, and discharged the same day.

Safety and effectiveness for closing multiple common femoral venous access sites per limb was demonstrated in a duplex ultrasound (DUS) IDE trial and three real-world investigator sponsored studies (ISS) with over 1,000 combined patients.
Click "Learn More" to find out more about these studies: Perclose Multi-Access Duplex Ultrasound (DUS) IDE Trial, Vascular Closure for Cardiac Ablation Registry (VACCAR), Emory School of Medicine (ESM) Study, Santa Barbara Cottage Hospital (SBCH) Study
The use of Perclose™ ProStyle™ Suture-Mediated Closure and Repair System can help:
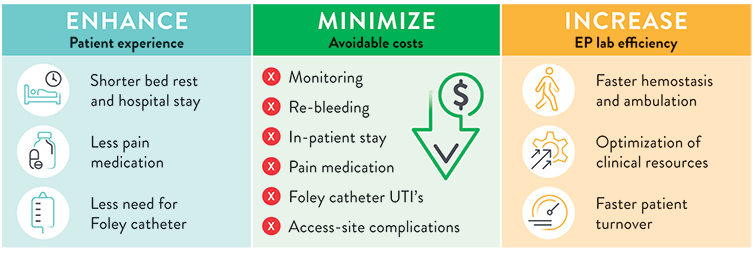
SOURCE: S. Verma. Adopting a Strategy of Early Ambulation and Same-Day Discharge for Atrial Fibrillation Ablation Cases - EP Lab Digest - May 2019.

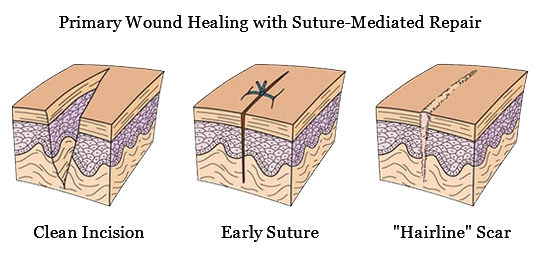
The Perclose™ ProStyle™ closure device achieves rapid hemostasis of femoral access sites by approximating the edges of the vessel wall with a surgical suture. The benefits of suture-mediated repair include promoting primary intention healing with less scarring14 and decreased time to hemostasis, ambulation, and patient discharge.15,16

Visit the official Perclose™ ProStyle™ website for more information on the features, deployment, clinical data, Perclose™ ProStyle™ videos, and ordering information related to Perclose™ ProStyle™ SMCR System.
¥ At the time of publication.
† Indicated for closing multiple access sites in the same common femoral vein.
*As compared to Angio-Seal‡, ExoSeal‡, Celt ACD‡, MANTA‡, Mynx‡, Vascade‡. Data on file at Abbott.
** after successful close with Perclose device(s) in patients who have undergone cardiac arrhythmia treatments with multiple common femoral venous access sites
***observed in a duplex ultrasound (DUS) IDE trial and two real-world investigator sponsored studies.
MAT-2004330 v7.0

Indications:
The Perclose™ ProStyle™ Suture-Mediated Closure and Repair System is indicated for the percutaneous delivery of suture for closing the common femoral artery and vein access sites of patients who have undergone diagnostic or interventional catheterization procedures.
The Perclose™ ProStyle™ SMCR System is indicated for closing the common femoral vein in single or multiple access sites per limb.
The Perclose™ ProStyle™ SMCR System is used without or, if required, with adjunctive manual compression.
For access sites in the common femoral artery using 5F to 21F sheaths. For arterial sheath sizes greater than 8F, at least two devices and the pre-close technique are required.
For access sites in the common femoral vein using 5F to 24F sheaths. For venous sheath sizes greater than 14F, at least two devices and the pre-close technique are required.
Caution:
Federal law restricts this medical device to sale by or on the order of a physician (or allied healthcare professionals, authorized by, or under the direction of, such physicians) who is trained in diagnostic and / or interventional catheterization procedures and who has been trained by an authorized representative of Abbott.
Prior to use, the operator must review the Instructions for Use and be familiar with the deployment techniques associated with the use of this device.
During closure of access sites using a procedural sheath greater than 8F, it is recommended that a vascular surgeon or a surgeon with vascular training be available in case surgical conversion to control bleeding and to repair the vessel is needed.
Contraindications:
There are no known contraindications to the use of this device.
Warnings:
Do not use the Perclose™ ProStyle™ SMCR System if the packaging or sterile barrier has been previously opened or damaged or if the components appear to be damaged or defective.
DO NOT RESTERILIZE OR REUSE. The Perclose™ ProStyle™ SMCR System is intended for single use only.
Do not use the Perclose™ ProStyle™ SMCR System if the sterile field has been broken where bacterial contamination of the sheath or surrounding tissues may have occurred, since such a broken sterile field may result in infection.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA) and / or above the inguinal ligament based upon bony landmarks, since such a puncture site may result in a retroperitoneal hematoma. Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Do not use the Perclose™ ProStyle™ SMCR System in arterial or venous access if the puncture is through the posterior wall or if there are multiple punctures in the same access site, since such punctures may result in a hematoma or retroperitoneal bleed.
Do not use the Perclose™ ProStyle™ SMCR System if the puncture site is located in the superficial femoral artery or the profunda femoris artery, or the bifurcation of these vessels, since such puncture sites may result in a pseudoaneurysm, intimal dissection, or an acute vessel closure (thrombosis of small artery lumen). Perform a femoral angiogram to verify the location of the puncture site. Note: This may require both a right anterior oblique (RAO) and left anterior oblique (LAO) angiogram to adequately visualize where the sheath enters the femoral vessel.
Precautions:
Potential Adverse Events:
Potential adverse events associated with use of vessel closure devices may include, but are not limited to, the following:
MAT-2100368 v4.0
Stay Connected