Brought to you only by Abbott, the AVEIR DR™ Dual Chamber Leadless Pacemaker (LP) System is first-of-its-kind technology that provides:
Our life-changing technology provides all the clinical benefits of traditional dual chamber pacemakers without the downsides. This means:
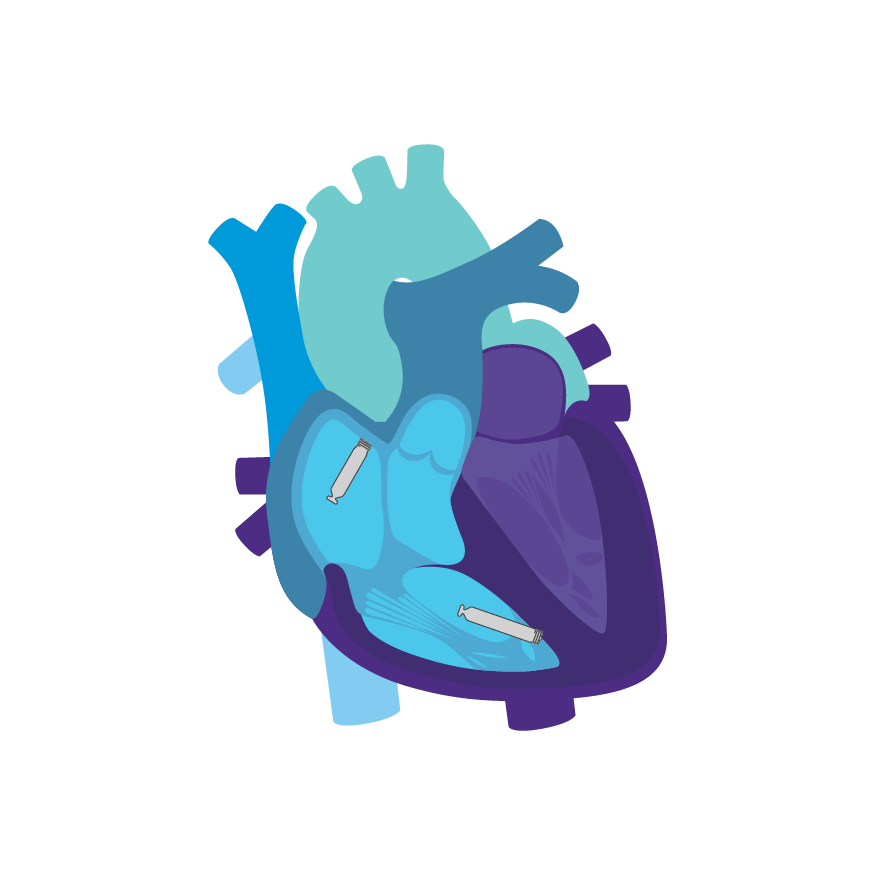
AVEIR™ DR Leadless Pacemaker System is capable of pacing and sensing in both chambers through the combination of an atrial leadless pacemaker (AVEIR™ AR LP) and a ventricular leadless pacemaker (AVEIR™ VR LP).
Dual chamber, leadless synchronous pacing between the atrium and the ventricle is made possible with industry-first, proprietary i2i™ communication technology capable of providing true DDD(R) pacing for continuous, atrioventricular (AV) synchrony, regardless of patient posture.

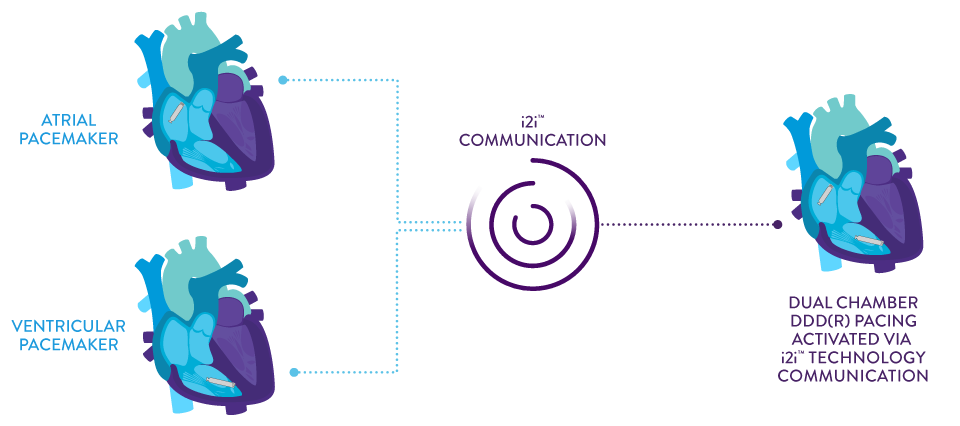
Patient therapy can be tailored by implanting an atrial or ventricular device alone, or both combined, for dual chamber support. The option to upgrade over time allows you to:
Treatment option 1: Start with the atrial device.
Treat sinus node dysfunction today.
Add a ventricular device for heart block later.

i2i™ communication is enabled.

Activate dual chamber pacing therapy DDD(R) via i2i Communication.
Treatment option 2: Start with the ventricular device.
Treat rare intermittent heart block today.

Add an atrial device for sick sinus syndrome later.

i2i communication is enabled.

Activate dual chamber pacing therapy DDD(R) via i2i communication.

This allows for the replacement of the atrial or ventricular device at end of service (EOS) without leaving hardware behind.
The AVEIR™ VR ventricular leadless pacemaker predicate device has a long-term retrieval success rate above 88% with helix fixation through 9 years regardless of implant duration.4


The specially designed retrieval catheter provides added confidence and is supported by a step-by-step retrieval protocol.5 AVEIR™ devices have an active fixation design that uses a screw-in mechanism to enable both implantation and long-term retrieval of the atrial and ventricular devices.2
Electrical mapping prior to fixation is designed to help reduce the number of repositioning attempts in the atrium and ventricle. AVEIR™ Leadless Pacemakers can assess Current of Injury via Commanded EGM, initial pacing capture thresholds, sensing amplitudes, and impedance in both atrial and ventricular chambers.
of patients had successful VENTRICULAR IMPLANTS WITH 1 OR LESS REPOSITIONING ATTEMPTS.3

of patients had successful ATRIAL IMPLANTS WITH 1 OR LESS REPOSITIONING ATTEMPTS.3


MAT-2406381 v1.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0