Diamondback 360™ is the only atherectomy system with a diamond-coated crown that orbits 360 degrees, specifically designed to target severe calcium.
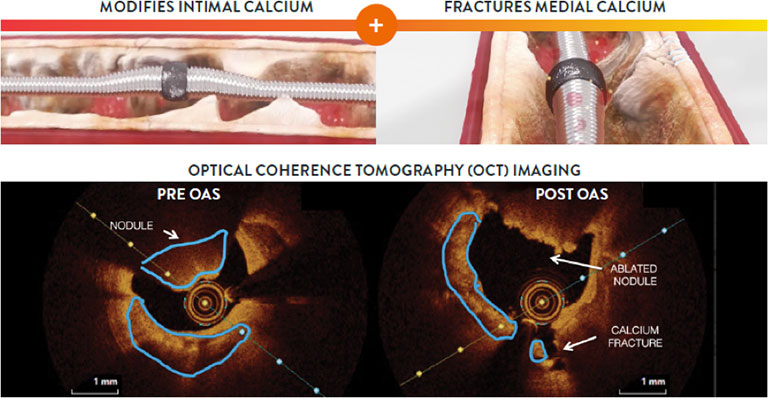
Diamondback 360™ Coronary Orbital Atherectomy System’s (OAS) unique dual mechanism of action uses differential sanding and pulsatile forces that enable simultaneous modification of both intimal and medial calcium for optimal stent delivery, expansion, and apposition in severely calcified lesions. One device treats eccentric, concentric, and nodular calcium.1-6
Diamondback 360™ OAS has a Unique Dual Mechanism of Action Design
One device reduces intimal calcium and fractures medial calcium. Differential sanding and pulsatile forces enable simultaneous modification of both intimal and medial calcium for optimal stent delivery, expansion, and apposition in severely calcified lesions.1-4
Watch video to learn more about the dual mechanism of action.
Diamondback 360™ OAS Gives You the Versatility to Treat Challenging Cases
Treat even the most severely calcified lesions, with under 2-minute setup7,8 and predictable procedure times.2
Facilitates antegrade and retrograde treatment of:
- Long, Diffuse Lesions
Successfully treated lesions up to 60 mm in length in real-world study.9 - Heavily Stenosed Lesions
Crossed >99% of lesions with <2% pre-dilatation in the ORBIT II study.1,10 - Nodular Lesions
Effectively treats nodular calcification.5,6 - Ostial Lesions
Safely treats ostial lesions.11-13
Low Profile
6F compatible for femoral or radial access.14
Multiple Vessel Sizes
A single 1.25 mm crown treats vessels 2.5 mm to 4.0mm14
Diamondback 360™ OAS Has Been Proven Effective and Safe in the Treatment of Severely Calcified Lesions.
Extensively studied, and with over 100,000 patients treated,15 orbital atherectomy has been demonstrated to perform effectively and safely in the treatment of severely calcified lesions.
>2,200
Patients Across 11 Robust Studies5,9
Proven Safety
<1%
Component Angiographic Complications in Two Real-world Studies9,16
Procedural Success
97.7%
Crossing and Stent Deployment in ORBIT II Study1
Low Q-Wave MI Rate
0.9%
In the ORBIT II Study at 30 days1
ORBIT II Target Lesions Revascularization (TLR)15
Sustained Clinical Performance
Data are for ORBIT II TLR in the OA+DES patient cohort.
3.4%
at 1 Year
5.1%
at 2 Years
6.6%
at 3 Years
The ORBIT II trial included patients with severely calcified lesions and demonstrated low rates of TLR at 1, 2, and 3 years.
References
- Chambers, J., et al., JACC Cardiovasc Interv. 2014;7(5):510-518.
- Shlofmitz, E., et al., Expert Rev Med Devices. 2017;14(11):867-879.
- Yamamoto, M., et al., Catheter Cardiovasc Interv. 2019;93(7):1211-1218.
- Kini, A., et al., Catheter Cardiovasc Interv. 2015;86(6):1024-1032.
- Shlofmitz, E., et al., Interv Cardiol. 2019;14(3):169-173.
- Abellas-Sequeiros, M., et al., REC Interv Cardiol. 2022;4(2):163-164.
- Lee, MS, et al., J Invasive Cardiol. 2016;28(4):147-150.
- Moses, J., Cardiac Interventions Today. 2015;May/June:75. Link accessed November 20, 2023. https://citoday.com/articles/2015-may-june/vesselpreparation-with-orbital-atherectomy-for-treating-severe-coronary-calcium-for-stent-delivery.
- Vinardell J, et al. J Am Coll Cardiol. 2020;76(17 Suppl S):B71.
- Data on file at Abbott. In the ORBIT II study, the OAS was inserted and activated in 434 subjects, but in 2 cases, the OAS was unable to cross the lesion.
- Lee MS, et al. J Interv Cardiol. 2018;31(1):15-20.
- Chambers JW, et al. Catheter Cardiovasc Interv. 2022;100(4):553-559.
- Ghazzal A, et al. Cardiovasc Revasc Med. 2024;58:52-57.
- Diamondback 360™ Coronary Orbital Atherectomy Systems Instructions for Use (IFU). Please refer to IFU for additional information.
- Data on file at Abbott.
- Lee, MS, et al., J Interv Cardiol. 2016;29(4):357-362.
MAT-2401825 v2.0
