Discover how the Perclose ProGlide™ System is clinically effective across a variety of procedures and specialties:
Objective: To compare the safety and effectiveness of the 'pre-close' technique for percutaneous femoral artery (FA) access and closure (PEVAR) to Surgical Cutdown (SEVAR).
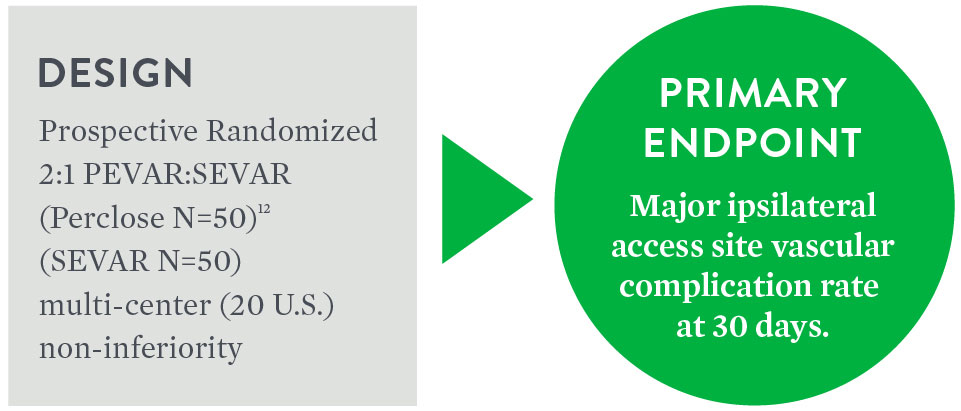
| PEVAR Perclose N=50 | SEVAR N=50 | Difference 95% CI3 | p-value3 | |
|---|---|---|---|---|
| Major Complications [95% CI]7 | 6% (3/50) [1.3%, 16.5%] | 10% (5/50) [3.3%, 21.8%] | -4.0% [-, 4.9%] | 0.0048 |
| PEVAR Perclose N=50 | SEVAR N=50 | p-value3 | |
|---|---|---|---|
| Minor Ipsilateral Access Site Vascular Complications at 30 Days [95% CI]7 | 4% (2/50) [0.5%, 13.7%] | 8% (4/50) [2.2%, 19.2%] | 0.67774 |
A prospective analysis was performed to evaluate the safety and effectiveness of Perclose™ Devices in closing large-sized venous access sites through a retrospective data collection. The prospective analysis included subjects in whom Perclose™ Devices were used as the primary method for large-bore venous access site closure during the TMVr index procedure with a 24F vascular sheath.
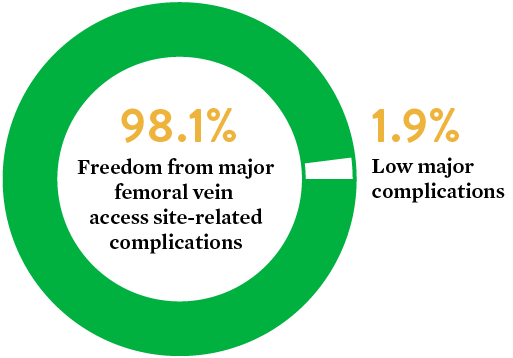
Source: The Use of the Perclose ProGlide™ Suture Mediated Closure (SMC) Device for Venous Access-Site Closure up to 24F Sheaths. Kar, Saibal; Hermiller, James; et al. CRT 2018.
9. EVEREST II/REALISM Continued Access Registry Study.
The Perclose™ Device vs. Surgical Cutdown retrospective study is designed to compare clinical outcomes and complication rates among patients undergoing closure of large-bore arterial access using Perclose™ Devices versus Surgical Cutdown (Cutdown) in a real-world setting.
The use of Perclose™ Devices for repair of large-bore arterial access is associated with significantly lower blood transfusions, infections, mortality, and length of stay compared to Surgical Cutdown.
| Cutdown | Perclose | |
|---|---|---|
| # of Patients | 757 | 757 |
| Anticoagulants◊ | 17.8% | 44.9% |
◊p<0.05
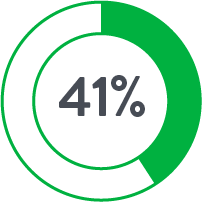
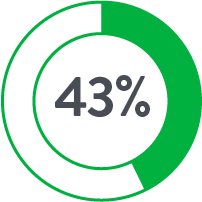
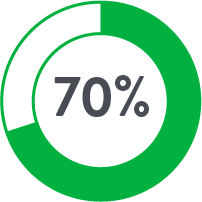
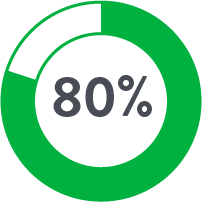
Source: Perclose ProGlide™ Versus Surgical Closure Outcomes – Real World Evidence. Schneider, Darren B; Krajcer, Zvonimir; et al. LINC 2018.
Objective: The aim of this publication was to report outcomes of endovascular aneurysm repair with percutaneous femoral access (PEVAR) using Prostar™ XL and Perclose™ Devices closure systems, from the multi-center Italian Percutaneous EVAR (IPER) registry.
10. Technical success was defined as the ability to obtain a successful percutaneous vascular access and closure without serious complications.
11. Major access-related complications included all the complications requiring an early surgical conversion due to bleeding, pseudoaneurysm, CFA stenosis/occlusion, and were considered a technical failure of system closure.
12. Minor access-related complications were related to the presence of minimal bleeding at the access site not requiring surgical conversion.
Objective: Assessment of efficacy, complication, and potential risk factors for Perclose™ Devices
Source: Saadi, Eduardo Keller, et al. "Totally Percutaneous Access Using Perclose ProGlide™ for Endovascular Treatment of Aortic Diseases." Brazilian Journal of Cardiovascular Surgery 32.1 (2017): 43-48.
13. Device failure defined as failure to achieve hemostasis at the access site, leading to alternative treatment other than manual compression.
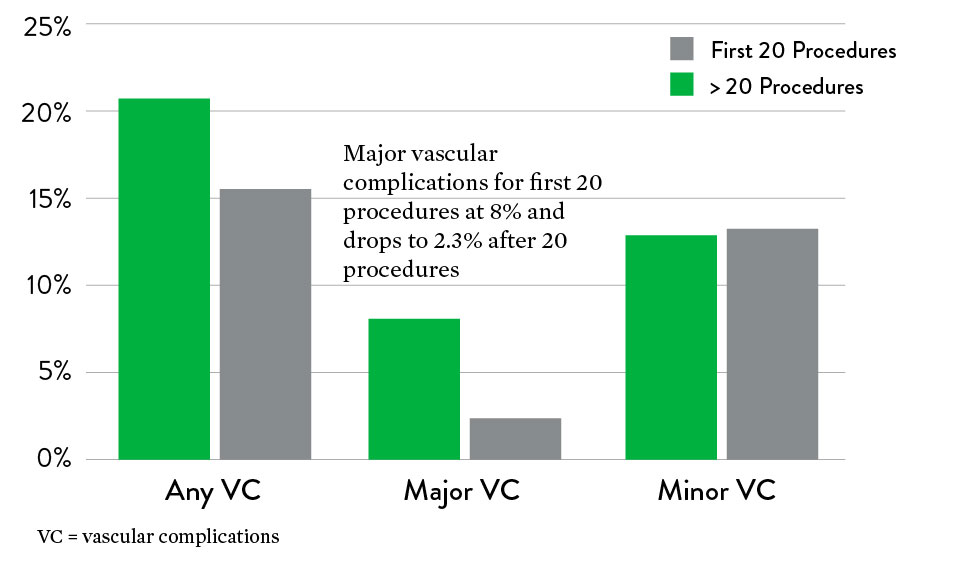
Objective: Evaluate the outcomes and predictive factors for additional Perclose™ Device deployments
14. Learning curve shown to be most pronounced over first 18 months. Bechara C.F. et al.: Predicting the learning curve and failures of total percutaneous endovascular aortic aneurysm repair. J Vasc Surg 2013; 57: pp. 72-76.
15. Primary technical success rate defined as the ability to achieve hemostasis without additional ProGlide™ device deployment or surgical repair.
16. Secondary technical success rate defined as the ability to achieve hemostasis after deployment of one or two ProGlide™ devices without surgical repair.
Objective: To evaluate the safety and effectiveness of the “pre-close” technique for PEVAR compared to surgical cutdown (SEVAR)
| Perclose PEVAR | SEVAR | p-value | |
|---|---|---|---|
| Major Complications | 6% (3/50) | 10% (5/50) | 0.0048 |
| Procedure Time | 106.5 ± 44.9 min | 141.1 ± 73.4 | 0.0056 |
| Time to Hemostasis | 9.8 ± 17.0 | 22.7 ± 22.9 | 0.00233 |
Source: Nelson, Peter R., et al. "A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial)." Journal of Vascular Surgery 59.5 (2014): 1181-1193.
17. Procedural technical success defined as successful vascular access and closure per randomized assignment, and successful endograft delivery, deployment, and catheter removal, without serious complication or need for vascular exposure in the percutaneous group.
Objective: Demonstrate clinical and cost benefits when utilizing a Fast-Track EVAR protocol with the Ovation stent graft system
| Fast Track | Standard EVAR*** | Fast-Track Savings | |
|---|---|---|---|
| Anesthesia | Local/Regional $300 | General $500 | $200 |
| Access | Bilateral PEVAR $1,200 | Cutdown† $300 | ($900) |
| ICU | 0% $0 | 1.4 Days, 51% $15,300 | $15,300 |
| Non-ICU | 1.2 Days $6,700 | 2.3 Days $12,900 | $6,200 |
| 30D Reintervention | 0% $0 | $29.4K, 1.1% $300 | $300 |
| Total | $8,200 | $29,300 | $21,100 |
**Standard EVAR Control group: Benchmarked 8,306 patients treated with standard, elective infrarenal EVAR at an alliance of ~3,750 U.S. Premier hospital facilities, based on Inpatient Discharge between 2012-2015.
***Standard EVAR: Average costs per patient, extracted costs for access, anesthesia, ICU, and hospital stay to calculate costs associated with Fast-Track.
†Assumes 30% applicability based on anatomic criteria, with 23% bilateral PEVAR and 7.0% unilateral PEVAR (Manunga et al., J Vasc Surg. 2013).
Source: Kracjcer, Z. Fast-Track Endovascular Aortic Repair: Final Results from the Prospective LIFE registry. VIVA 2016.
18. Technical Success (Successful Bilateral PEVAR)
Objective: To evaluate the efficacy of Perclose™ Devices
Source: Barbash, Israel M., et al. "Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation." European Heart Journal 36.47 (2015): 3370-3379.
Objective: Evaluate the safety and efficacy of Perclose™ Devices
Source: Seeger, Julia, et al. “Impact of suture mediated femoral access site closure with the Prostar XL compared to the Perclose system on outcome in transfemoral aortic valve implantation.” International Journal of Cardiology 223 (2016): 564-567.
19. Device failure defined as failure to achieve hemostasis at the access site leading to alternative treatment other than manual compression. 4.6% (n=16) needed stenting for device failure.
Objective: Assessment of efficacy, complication, and potential risk factors for Perclose™ Devices
Source: Saadi, Eduardo Keller, et al. "Totally Percutaneous Access Using Perclose ProGlide™ for Endovascular Treatment of Aortic Diseases." Brazilian Journal of Cardiovascular Surgery 32.1 (2017): 43-48.
20. Device failure defined as failure to achieve hemostasis at the access site, leading to alternative treatment other than manual compression.
Safety and performance of Perclose ProGlide™ vascular closure device in managing large-hole venous access site
Objective: The primary objective of this study was to evaluate the safety and performance of ProGlide™ in the closure of the venous access site in subjects treated with a large-caliber femoral vein sheath (24F).
MAT-2414807 v1.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0