Real-World Evidence Demonstrates Leadless Pacing Safety Data
Abbott Cardiac Rhythm Management | November 12, 2024

This real-world evidence (RWE) from a U.S. nationwide study shows that leadless pacemakers prevent lead complications and chest pocket infections while also providing data that prove safety benefits over traditional pacemakers.
This RWE is the first commercial study comparing transvenous VVI(R) implants with AVEIR™ VR Ventricular Leadless Pacemaker (LP) implants in U.S. Medicare patients. The results shown here represent adjusted event rates which account for baseline differences between LP and transvenous to make fair comparisons while more closely reflecting the effect of the devices on outcomes.
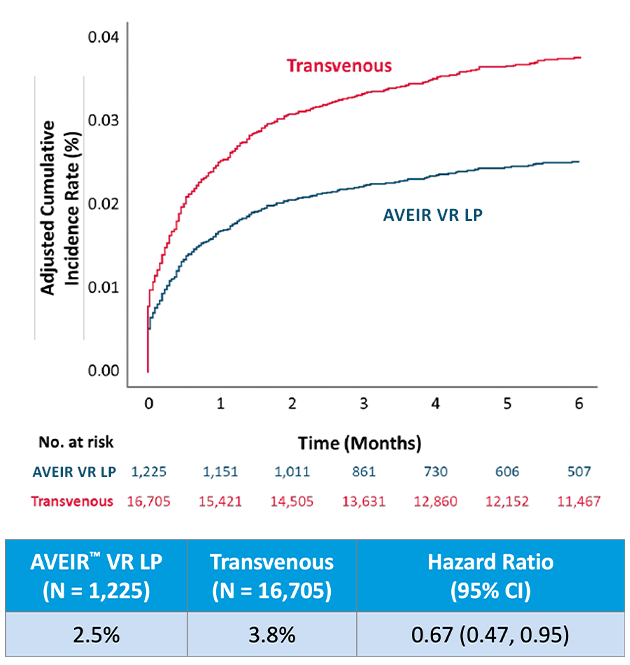
The table below shows overall AVEIR VR LP chronic complication rates, trending lower at 6 months1,3 but not statistically significant compared to transvenous VVI(R).

Here, the AVEIR VR LP has a 33% lower risk of chronic device-related complications compared to transvenous VVI(R) pacemakers at 6 months.1,3
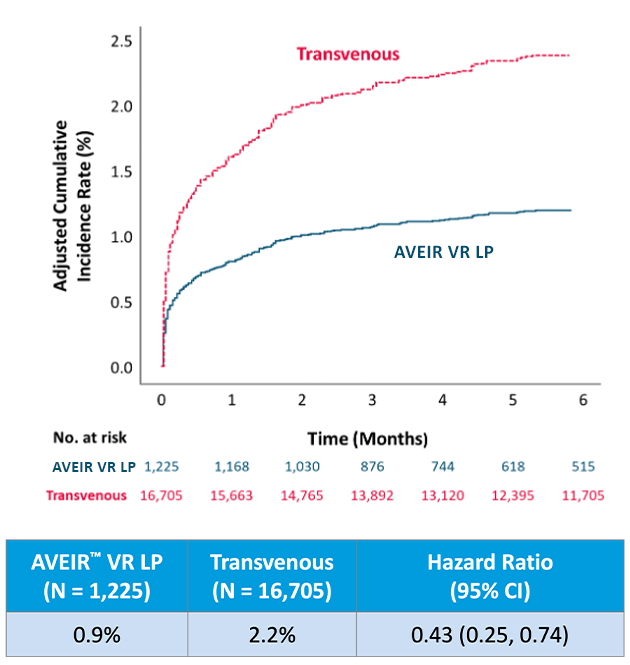
Looking at device reintervention rate at 6 months,1,3 the AVEIR VR LP has a 57% lower risk of reinterventions compared to transvenous VVI(R) pacemakers.
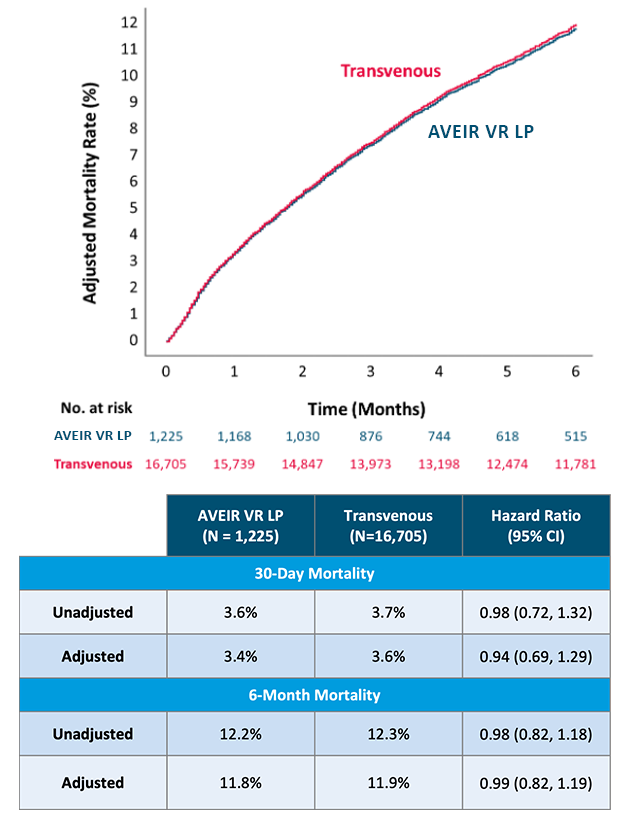
Compiled data looking at the all-cause mortality rates1,3 showed no significant difference in mortality between AVEIR VR LP and transvenous VVI(R) pacemakers at 30 days and 6 months.
Data also shows that the 30-day and 6-month individual complications1 showed no significant difference in cardiac effusion/perforation at 30 days.

Overall, the AVEIR VR LP improves outcomes for patients with significant bradycardia and normal sinus rhythm, with rare episodes of AV block or sinus arrest, as well as those with chronic atrial fibrillation. Stay tuned for a future blog post with more data on dual chamber leadless pacing from this study.
In addition to the significant benefits of leadless pacemakers over transvenous ones highlighted here, you can find more data on AVEIR VR LPs, including long-term retrieval, battery longevity, and dual chamber leadless pacing, by visiting the website. here.
REFERENCES:
- Ip J, Brady P, et al. Leadless vs. Transvenous Single-Chamber Ventricular Pacemakers: Real-World Evidence from AVEIR™ VR Coverage with Evidence Development Study. Poster presented at: HRS 2024; May 17, 2024, Boston, MA.
- Knops R, Ip J, et al. One year safety and performance outcomes from a clinical study of a dual-chamber leadless pacemaker system. Heart Rhythm. 2024;21(7):1199-1200. https://doi.org/10.1016/j.hrthm.2024.04.026
- Reddy V, et al. How Safe is Aveir? Data Summary from Aveir DR IDE and VR Real-World Evidence Studies. Abstract Presented at: HRS 2024; May 18, 2024, Boston, MA.

MAT-2412540 v1.0