
We’re excited to introduce the world’s first dual chamber leadless pacemaker system that has now been approved by the U.S. Food and Drug Administration (FDA). The AVEIR™ DR Dual Chamber Leadless Pacemaker System is the only leadless solution that provides therapy for all common pacemaker indications, which is made possible by the world’s first atrial leadless pacemaker.
View the video below to discover more about this revolutionary technology.
Until now, leadless pacing could be done only in the right ventricle to treat patients with significant bradycardia with normal sinus rhythm and only rare episodes of AV block or sinus arrest and chronic atrial fibrillation. This VVI group represents 19% of all pacemaker patients.1 Because of this new approval and launch, now all your patients can benefit from leadless pacing that eliminates lead- and pocket-related complications and offers no visible scarring or arm movement restrictions.
The AVEIR DR Dual Chamber Leadless Pacemaker System consists of two individual leadless pacemakers: one for the right atrium and the other for the right ventricle.1 These two devices can operate alone or together.
Based on your patient’s symptoms, you can implant an atrial or ventricular AVEIR Leadless Pacemaker and add the other one later for dual chamber pacing. The flexibility of the system lets you tailor therapy to your patient’s specific needs when they need it. Additionally, long-term retrievability allows for the replacement of the atrial or ventricular device at end of service without leaving hardware behind.2
The flexibility to tailor therapy is made possible by our proprietary implant-to-implant (i2i™) communication technology. Employing high-frequency pulses to relay messages via the naturally conductive characteristics of the body, this technology allows the pacemakers to establish continuous beat-to-beat atrioventricular synchrony.
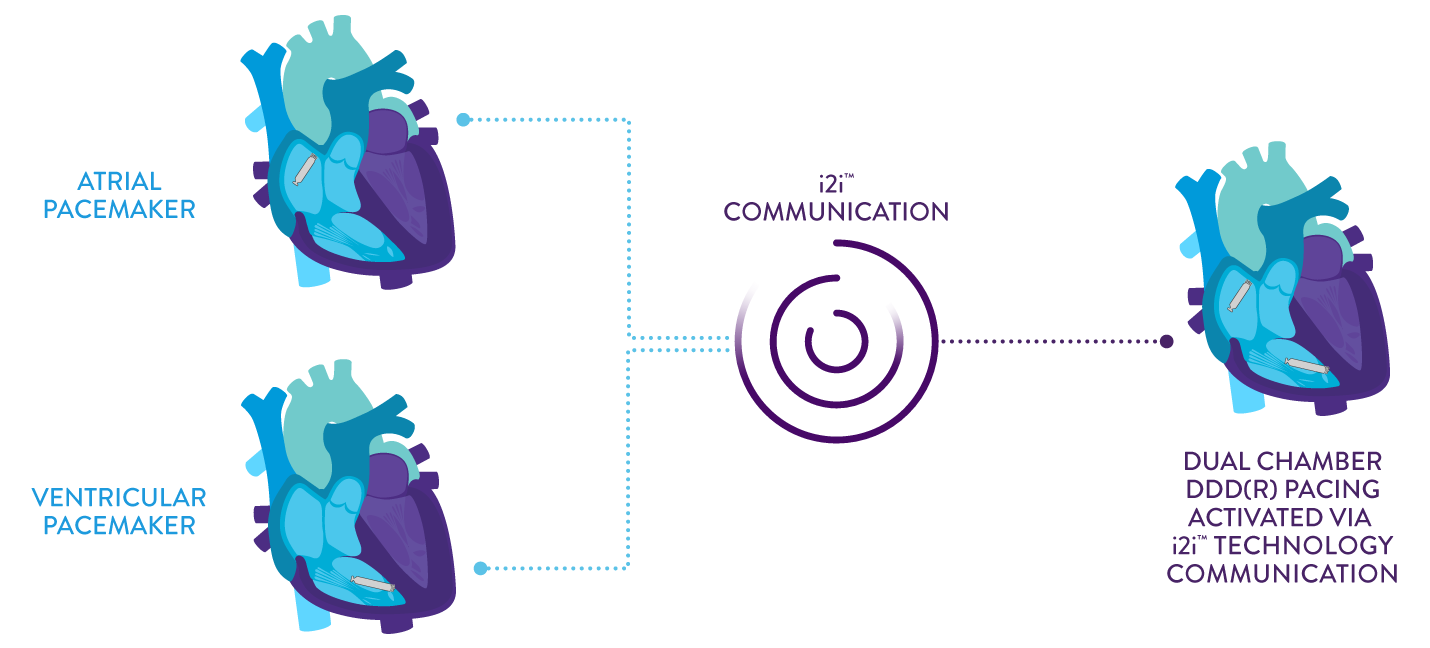
With the ability to pace and sense in both the right atrium and right ventricle, you now can offer leadless pacing to treat patients with sick sinus syndrome; chronic, symptomatic second and third-degree AV block; and symptomatic bilateral bundle-branch block when tachyarrhythmia and other causes have been ruled out.
See how the minimally invasive procedure can change patient lives like Cecilia Lee in Virginia who was implanted with the AVEIR DR Dual Chamber Leadless Pacemaker System a year ago. Watch as Cecilia discusses her story.
In our worldwide clinical trial, the results of the first 300 patients were used to calculate endpoints for the AVEIR DR i2i™ IDE Study and showed ≥95% mean AV synchrony for multiple postures and gaits (including sitting, supine, left lateral recumbent, right lateral recumbent, standing, normal walking and fast walking).3 These never-before-seen results from a leadless pacemaker system proves the benefit of this proprietary i2i technology and how it can solve for your patients’ needs.
Learn more about what the AVEIR DR Dual Chamber Leadless Pacemaker System can do for your practice by visiting the AVEIR™ LP product page or view the press release announcing its FDA approval.
REFERENCES
Please read the Important Safety Information.


MAT-2311028 v2.0
Stay Connected