Unveiling the i2i™ Communication Technology in the AVEIR™ DR Leadless Pacemaker (LP) System
Abbott Cardiac Rhythm Management | December 14, 2023

In the world of cardiac care, the advent of leadless pacemakers has been a groundbreaking leap. The AVEIR™ DR LP System, housing two leadless pacemakers, orchestrates a symphony within the heart through an innovative communication technology known as i2i™ (implant-to-implant) communication. In a conversation with Dr. Matthew Fishler, the Director of Systems Engineering and Chief Engineer for the Leadless Platform, we delve into the intricacies of i2i technology and its role in shaping the future of cardiac pacing.
Q: How do the leadless devices communicate, and what makes i2i unique?
A: The AVEIR DR LP System employs a novel i2i communication paradigm, where the leadless pacemakers in the right atrium (RA) and right ventricle (RV) engage in bidirectional communication, i.e., on a beat-to-beat basis. Unlike traditional wireless methods, i2i utilizes conducted electrical signaling akin to electrical Morse code. This method provides advantages, such as low power consumption, leveraging existing electrodes, and enhanced security, due to the signals being confined within the patient's body.
Q: What challenges were overcome in developing i2i technology?
A: Embarking on a groundbreaking frontier in medical technology, we faced an unprecedented challenge: orchestrating the seamless collaboration of two independent leadless pacemakers as a singular, coordinated system—an achievement that, until now, had not seemed possible in the realm of implantable devices. The solution emerged in i2i communication, which offers energy efficiency and robust safety, maintaining rhythm for the patient even if communication is disrupted. The technology is a revolutionary paradigm that not only defies the conventional, but stands as the cornerstone of the AVEIR DR LP System.
Q: What factors could impact i2i communication and how does it adapt to individual patient variations?
A: Many factors—including heart size, device positioning within the heart, and patient posture, to name a few—can impact the robustness of i2i communication between leadless pacemakers since conducted signaling depends on both the relative distance and relative angles between the devices. Our i2i technology accommodates variations in distance and angles between the leadless pacemakers and is intentionally designed to suit a range of patient factors. The strength of i2i signals can be adjusted based on measurements of i2i functionality and additional diagnostics obtained during programming sessions, providing customization for each patient.
Q: How does i2i ensure a long lifespan without frequent battery replacements?
A: i2i's power-efficient communication relies on conducted electrical signaling through the bloodstream. Strategies, like low-frequency wake-up pulses and simplified message variants, optimize power consumption, ensuring a longer lifespan without frequent battery changes. The wake-up pulses initiate communication: a "low-frequency" pulse activates an always-on low-power receiver in the remote LP, followed by a higher power receiver for the subsequent "high-frequency" message. The message itself comes in "simple" (9 essential bits) or "extended" (32 bits) variants. Most of the time, the efficient "simple" variant is used, with occasional switches to the more detailed "extended" variant for specific needs like rate changes or mode switches. This meticulous approach maximizes power efficiency while maintaining full dual chamber functionality.
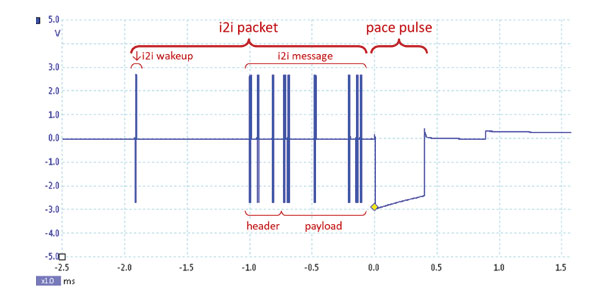
Q: What safety features mitigate risks?
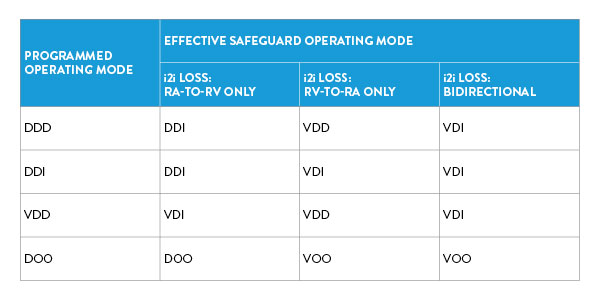
A: Recognizing the inherent challenges in maintaining seamless communication within any system, particularly one as critical as the AVEIR DR LP System, several core design features have been integrated to proactively address potential disruptions and ensure the patient’s well-being. In adopting the "Parent/Child" functional design modality, where the RV device assumes the role of the “Parent” and the RA device that of the “Child,” the system guarantees continuous ventricular pacing, even in the event of i2i communication disruptions. Operating modes are fortified with dynamic safeguards, adapting seamlessly to i2i losses, while the "i2i Vigilance Control" intelligently prevents asynchronous pacing through adept atrial pacing management. The subsequent table provides a concise summary of each programmed dual chamber operating mode's transition to an effective safeguard operating mode based on the directionality of i2i communication loss:
Q: How did interdisciplinary collaboration contribute to i2i development?
A: The collaboration between engineers, clinicians, and specialists was pivotal in ideation, design, and validation to address concerns and validate prototypes. A memorable moment occurred during an early preclinical trial where a clinician witnessed the two leadless pacemakers operating in dual chamber mode for the very first time in an actual beating heart. After seeing the successful communication occur, the clinician knew this i2i technology would revolutionize the field.
Q: What insights into clinical validation processes can you share?
A: As a groundbreaking technology, the AVEIR DR LP System was characterized and tested rigorously before being implanted into any patients. This included extensive simulated-use bench-level assessments of system behavior under normal and challenging conditions; numerous feasibility and formal chronic preclinical validation studies; and a suite of formal human factors usability validation studies with anticipated end-users, including clinicians, field reps, nurses, and technicians.
Q: Any ongoing developments in i2i technology?
A: The success of the first-generation i2i technology has sparked enthusiasm for further improvements. The cross-functional team is actively working on the next generation of the i2i protocol.
Interested in learning more about the revolutionary technology behind the AVEIR DR LP System? Explore more on the AVEIR DR LP product page.
™ Indicates a trademark of the Abbott group of companies.


MAT-2314655 v2.0
Stay Connected