The Volt PFA System is an investigational devices. LIMITED BY FEDERAL (OR U.S.) LAW TO INVESTIGATIONAL USE. This product is not available for sale in the United States.
Before being used with patients, the Volt PFA System underwent extensive laboratory testing. Following this, the Volt CE Mark Study was the first clinical research study to investigate the Volt PFA System's safety and performance in treating human patients. The purpose of this study was to gather data demonstrating that the Volt PFA System functions as intended in a clinical setting and to establish its safety and effectiveness for treating atrial fibrillation.

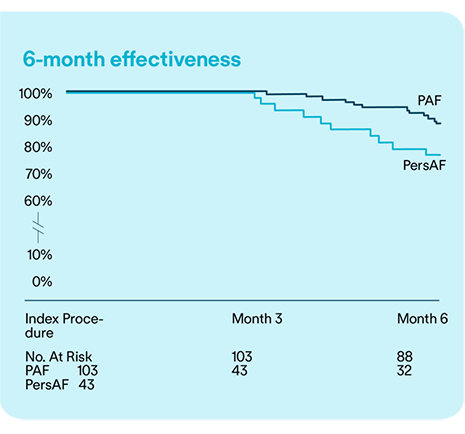
Safety and Effectiveness of balloon-based PFA system for de novo PVI to treat PAF and PersAF: 6-Month Results of the Volt CE Mark Study.
Data from the Volt CE Mark study demonstrates that PVI with the Volt™ PFA System is both safe and effective for treating PAF and PsAF over a 6-month follow-up period.1
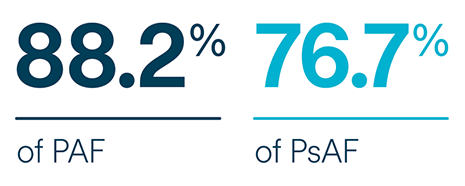
subjects free from documented AF/AFL/AT recurrence at 6-months
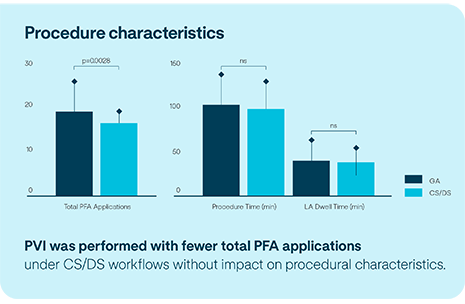
Acute safety and procedural characteristics of conscious and deep sedation compared to general anesthesia workflows with novel balloon-based PFA system.
This sub-analysis of the CE Mark data, presented by Prof. Roland Tilz, demonstrates that PVI with the Volt™ PFA Catheter, Sensor Enabled™ in subjects under deep or conscious sedation (CS/DS) does not significantly impact safety or acute outcomes compared to procedures with general anesthesia (GA).2
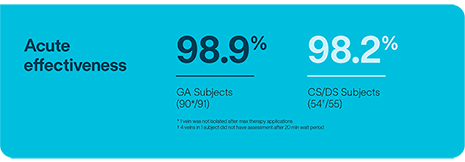
In the Volt CE Mark feasibility sub-study, acute effectiveness was achieved in 99.2% (127/128) of treated PVs (96.9% of subjects, 31/32) with 23.8 ± 4.2 PFA applications/subject. No esophegeal lesions causally related to Volt™ PFA System.
This clinical research study is intended to demonstrate safety and effectiveness of the Volt™ PFA System for the treatment of symptomatic, recurrent, drug-refractory paroxysmal and persistent atrial fibrillation.
2024 Manuscript Assessing PFA Design Considerations
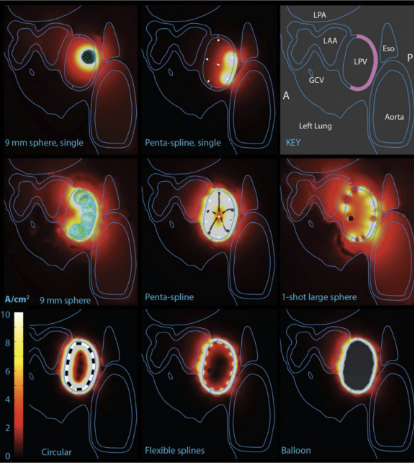
Comparison of efficiency of PFA catheter designs by computer modeling. Computer models demonstrate a wide range in efficiency among PFA catheters. Form factors such as exposure of PFA electrodes to blood pool significantly influence efficiency. Higher efficiency designs, such as balloon-based designs, have less collateral current.
The Volt PFA System is an investigational devices. LIMITED BY FEDERAL (OR U.S.) LAW TO INVESTIGATIONAL USE. This product is not available for sale in the United States.
MAT-2500133 v5.0
Stay Connected