Experience the power of next-generation technology with our newest addition to your One System Solution: Advisor™ HD Grid X Mapping Catheter, Sensor Enabled™. This advanced tool is now better than ever, offering a boost in accuracy, speed, and versatility1.
We’ve made advancements to our novel first-generation Advisor™ HD Grid Mapping Catheter, Sensor Enabled™ - which offered a first-of-its-kind electrode configuration for high-density mapping, allowing you to place 16 electrodes where you need them. Our unique first-generation design platform, Advisor HD Grid Mapping Catheter, SE was designed to maneuver bi-directionally within all chambers of the heart.
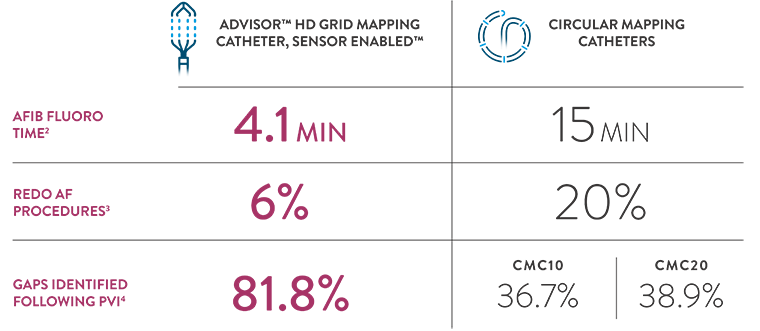
Leveraging our legacy with the Advisor HD Grid Mapping Catheter, SE, we returned to our roots, building on our foundation and drawing strength from our past. The Advisor HD Grid Mapping Catheter, SE reduces radiation2, redo AF procedures3, and identifies gaps often missed by circular mapping catheters4.
The innovative Advisor HD Grid X Mapping Catheter, SE leverages the advanced capabilities of the EnSite X, compatible with EnSite™ VoXel Mode through its 2 additional sensors. When paired with the EnSite™ X, the catheter’s grid-pattern displays true*, localized signals often missed by traditional mapping catheters.
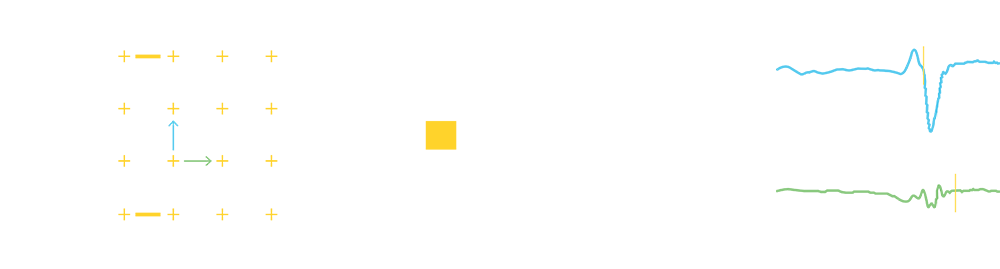
The addition of two paddle sensors to the new Advisor HD Grid X collects data faster1, create more accurate unedited models1, and enable you to map with high confidence1 in any cardiac chamber**.
*EnSite Omnipolar Technology captures true signals independent of catheter orientation relative to the wavefront.
**Supported by data from a pre-clinical animal study involving 6 subjects. Results are not necessarily indicative of clinical performance.
A preclinical study with the objective of comparing Advisor HD Grid X Mapping Catheter, SE to Octaray and Advisor HD Grid Mapping Catheter, SE. Four participating independent physicians performed the study protocol with each of the HD Grid X Mapping Catheter, SE, Octaray, and Advisor HD Grid Mapping Catheter, SE catheters within the atria and ventricles of each of six swine study subjects. The protocol measured each catheter on (1) mapping and modeling speed; (2) model accuracy; and (3) ectopic burden

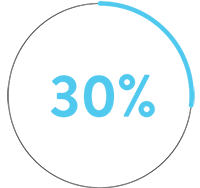
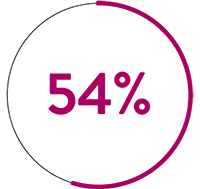
In a pre-clinical, head-to-head comparison, Advisor HD Grid X Mapping Catheter, SE beats Octaray‡1 in 3 key areas:
than Octaray‡1
than Octaray‡1
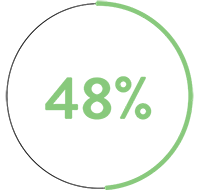
than Octaray‡1
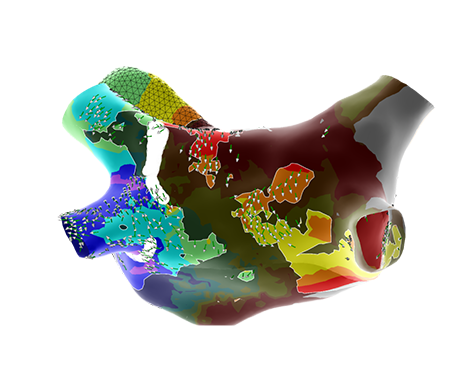
*Supported by data from a pre-clinical animal study involving 6 subjects. Results are not necessarily indicative of clinical performance. A preclinical study with the objective of comparing Advisor HD Grid X Mapping Catheter, SE to Octaray and Advisor HD Grid Mapping Catheter, SE. Four participating independent physicians performed the study protocol with each of the HD Grid X Mapping Catheter, SE, Octaray, and Advisor HD Grid Mapping Catheter, SE catheters within the atria and ventricles of each of six swine study subjects. The protocol measured each catheter on (1) mapping and modeling speed; (2) model accuracy; and (3) ectopic burden
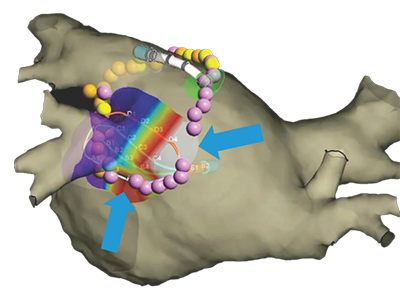
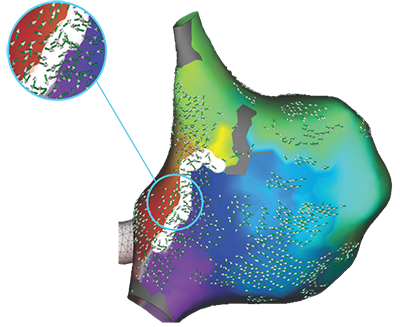
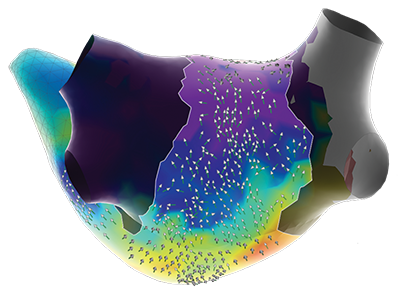
*EnSite Omnipolar Technology captures true signals independent of catheter orientation relative to the wavefront

*Supported by data from a pre-clinical animal study involving 6 subjects. Results are not necessarily indicative of clinical performance.
**Every signal can be defined as any signal seen on the RAI window recorded by the Advisor HD Grid Mapping Catheter, SE when the map polarity is set to omnipolar.
References:
Information contained herein is intended for distribution outside the U.S. only, excluding Finland.
MAT-2413534 v1.0
Rx Only. Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events, and directions for use.
United States: Required Safety Information
Indications for Use: The Advisor™ HD Grid X Mapping Catheter, Sensor Enabled™, is indicated for multiple electrode electrophysiological mapping of cardiac structures in the heart, i.e., recording or stimulation only. This catheter is intended to obtain electrograms in the atrial and ventricular regions of the heart. Contraindications: The catheter is contraindicated for patients with prosthetic valves, and patients with left atrial thrombus or myxoma, or interatrial baffle or patch via transseptal approach. This device should not be used with patients with active systemic infections. Patients unable to receive heparin or an acceptable alternative to achieve adequate anticoagulation. Warnings: Persons with a known history of allergies to any of the materials listed below may suffer an allergic reaction to this device. Before use, counsel the patient on the materials contained in the device and discuss a thorough history of allergies. This device contains: Acrylonitrilebutadienestyrene (ABS Cycolac) – Loctite Adhesive – Pellethane – Platinum Iridium alloy – Polyimide – Polyether block amide (PEBAX) – Polyethylene (High Density Polyethylene, HDPE) – Titanium. Cardiac catheterization procedures present the potential for significant xray exposure, which can result in acute radiation injury as well as increased risk for somatic and genetic effects, to both patients and laboratory staff due to the xray beam intensity and duration of the fluoroscopic imaging. Careful consideration must therefore be given for the use of this catheter in pregnant women. The safety and effectiveness of the device has not been established in pregnant women or prepubescent children. Careful consideration must therefore be given for the use of the device in pregnant women or prepubescent children. Catheter entrapment within the heart or blood vessels is a possible complication of electrophysiology procedures. Vascular perforation or dissection is an inherent risk of any electrode placement. Careful catheter manipulation must be performed in order to avoid device component damage, thromboembolism, cerebrovascular accident, cardiac damage, perforation, pericardial effusion, or tamponade. Risks associated with electrical stimulation may include, but are not limited to, the induction of arrhythmias, such as atrial fibrillation (AF), ventricular tachycardia (VT) requiring cardioversion, and ventricular fibrillation (VF). Do not use force to advance or withdraw catheter when resistance is encountered. Do not immerse the proximal handle or cable connector in fluids; electrical performance could be affected. Precautions: Maintain an activated clotting time (ACT) of greater than 300 seconds at all times during use of the catheter. This includes when the catheter is used in the right side of the heart. To prevent entanglement with concomitantly used catheters, use care when using the catheter in the proximity of the other catheters. Maintain constant irrigation to prevent coagulation on the distal paddle. Inspect irrigation tubing for obstructions, such as kinks and air bubbles. If irrigation is interrupted, remove the catheter from the patient and inspect the catheter. Ensure that the irrigation ports are patent and flush the catheter prior to reinsertion. Use the straightener during the insertion process to avoid damage to the hemostasis valve. Always straighten the catheter before insertion or withdrawal. Catheter advancement must be performed under fluoroscopic guidance to minimize the risk of cardiac damage, perforation, or tamponade. Compatible navigation and real time visualization systems may also be considered. Do not use if the catheter appears damaged, kinked, or if there is difficulty in deflecting the distal section to achieve the desired curve. Catheter materials are not compatible with magnetic resonance imaging (MRI). One or more components of this device may contain the following substance defined as CMR 1B in a concentration above 0.1% weight by weight: Nmethyl2pyrrolidone (NMP): Chemical Abstracts Service (CAS) No. 872504; EC No. 2128281. Based on a quantitative toxicological assessment it has been determined that NMP released from this device is unlikely to cause adverse reproductive effects. Potential Adverse Events: Complications related to the use of the device include, but are not limited to, the following: New or worsening of existing arrhythmia including Atrial fibrillation, Ventricular tachycardia requiring cardioversion, Supraventricular tachycardia (SVT) and Ventricular fibrillation Cardiac perforation including Pericardial effusion or Cardiac tamponade Bleeding including access site Hemorrhage / bleeding, Ecchymosis and Hematoma Vascular access complications or peripheral vascular injury including Femoral artery dissection, Dissection, Arteriovenous fistula and Pseudoaneurysm formation Pulmonary vein stenosis, Heart failure, Volume overload, Hypotension, Embolism, Cerebrovascular accident (CVA)/stroke, Infection, Pneumonia, Pulmonary edema, Immunological reaction, Pain and Pericarditis.
MAT-2503929 v1.0
Stay Connected