Coronary calcium tends to be underestimated. Calcium considered mild or moderate by angiography may actually be severe if advanced imaging such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT) imaging are used.1,2 Moderate to severe arterial calcium is present in 30-40% of patients who undergo a PCI.2,3
Challenges Associated with Calcium4
Our newest coronary orbital atherectomy system is built on our proprietary Diamondback 360™ platform and enhanced with a sheathed inner driveshaft designed to deliver better 1:1 treatment motion for smoother, more efficient, treatment in severe calcium.*
* Compared to previous generation Diamondback 360 OAD. Data on file at Abbott. Based on cadaveric testing. Non-clinical bench testing results may not be indicative of clinical performance.
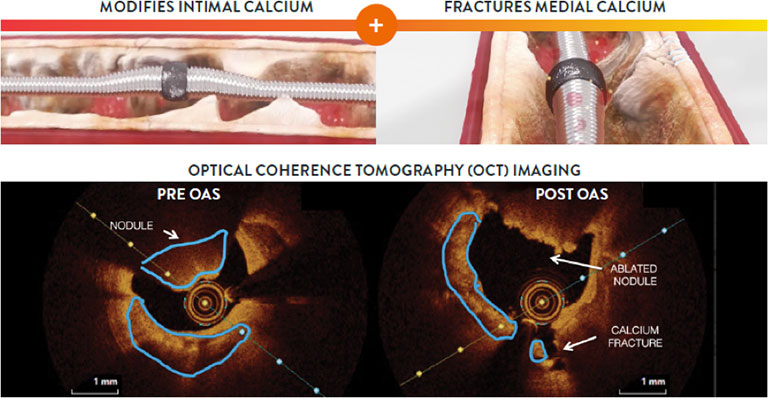
Orbital Atherectomy reduces intimal calcium and fractures medial calcium. Differential sanding and pulsatile forces enable simultaneous modification of both intimal and medial calcium for optimal stent delivery, expansion, and apposition in severely calcified lesions.3-8
Facilitates antegrade and retrograde treatment of:
6F compatible for femoral or radial access.
Single device treatment of multiple lesions and vessel sizes.
Extensively studied, and with over 100,00014 patients treated, orbital atherectomy has been demonstrated to perform effectively and safely in the treatment of severely calcified lesions.3 See more details.
†United States Patent #: 10,299,820
MAT-2310616 v2.0

Including the Orbital Atherectomy Device (OAD) with GlideAssist™, Saline Pump, ViperWire Advance™ Coronary Guide Wire, and ViperWire Advance™ with Flex Tip Coronary Guide Wire
INDICATIONS
The Diamondback 360™ Coronary Orbital Atherectomy System (OAS) is a percutaneous orbital atherectomy system indicated to facilitate stent delivery in patients with coronary artery disease (CAD) who are acceptable candidates for PTCA or stenting due to de novo, severely calcified coronary artery lesions.
CONTRAINDICATIONS
Use of the OAS is contraindicated for use in the following situations:
WARNINGS
PRECAUTIONS
POTENTIAL ADVERSE EVENTS
Potential adverse events that may occur and/or require intervention include, but are not limited to:
Diamondback 360™ and Diamondback 360 Precision™ Coronary Orbital Atherectomy System are manufactured and distributed by Cardiovascular Systems, Inc. (CSI). CSI is a subsidiary of the Abbott Group of Companies.
MAT-2303956 v1.0
Stay Connected