Our lead portfolio is built on years of proven reliable lead technology. We design for deliverability, stability and performance.
Introducing the UltiPace Pacing Lead, newly engineered from Abbott, the first and only provider of stylet-driven leads approved for left bundle branch area pacing (LBBAP) with a robust distal lead tip design.* LBBAP performance features include resistance to distal fatigue failure,1 zero helix retraction1 when used with the Helix Locking Tool, and improved torque transmission.1 An enhanced proximal lead end provides increased abrasion and lead crush resistance with 6F compatibility.2

Designed to offer high-performance handling and more control at implant, the Durata Defibrillation Lead is a cardiac lead that has a proven structural design for reliability and long-term durability.3 The Durata Defibrillation Lead is designed to deal with challenging ICD lead cases, including cases requiring multi-lead systems, lead replacement or with patients who have small or tortuous veins.
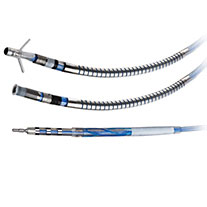
The Quartet quadripolar LV lead offers superb deliverability with exceptional stability and performance. With four electrodes and up to 14 pacing configurations, the quadripolar system enables left ventricular (LV) pacing at the preferred site without compromising lead stability.4
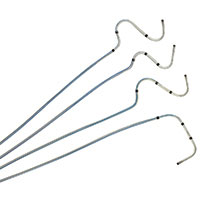
The Tendril STS Pacing Lead has helped to improve patient health and restore quality of life to thousands across the globe. Compatible with a 6F introducer, the Tendril™ STS Pacing Lead has a thin profile, soft silicone tip and abrasion-resistant Optim™ lead insulation.5

*Robust distal lead tip without a flexible zone in distal 14.5 mm. CE and FDA Approval for LBBAP.
MAT-2012194 v3.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0