Volt™ PFA System simplifies therapy delivery, minimizing procedural burden so you can treat more patients with ease and precision.1*
Delivers targeted lesion sets where it matters with an 8-spline balloon-in-basket design that conforms to anatomy and creates a wide-band lesion2 for fewer repositions
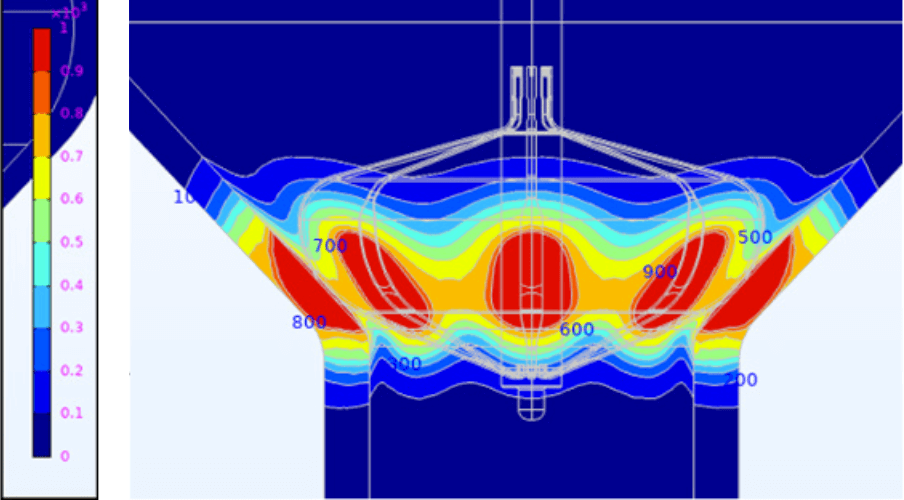
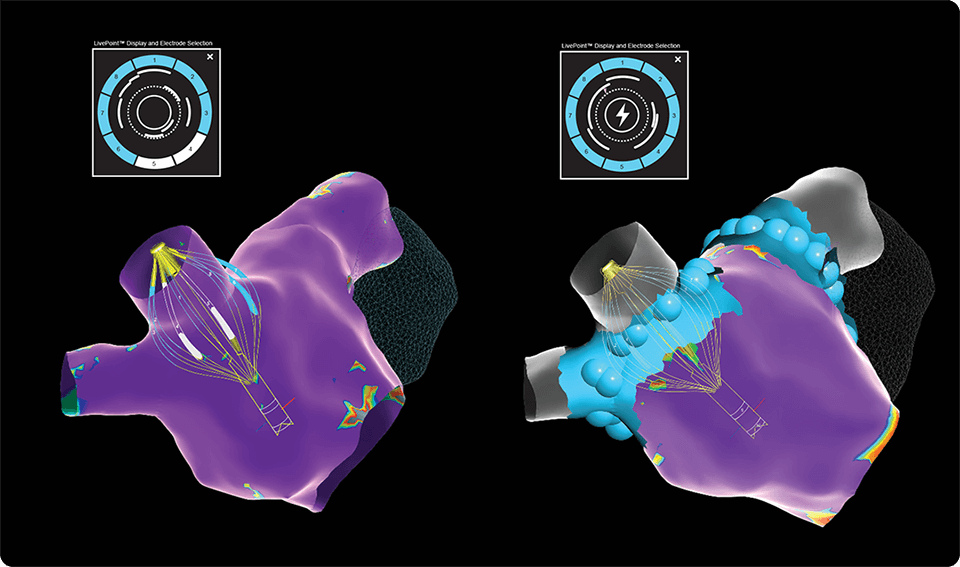
Gain real-time procedural insight with elegant impedance-based contact visualization through AutoMark and eField integration for precise lesion tracking3,4
Three handling options for optimal positioning



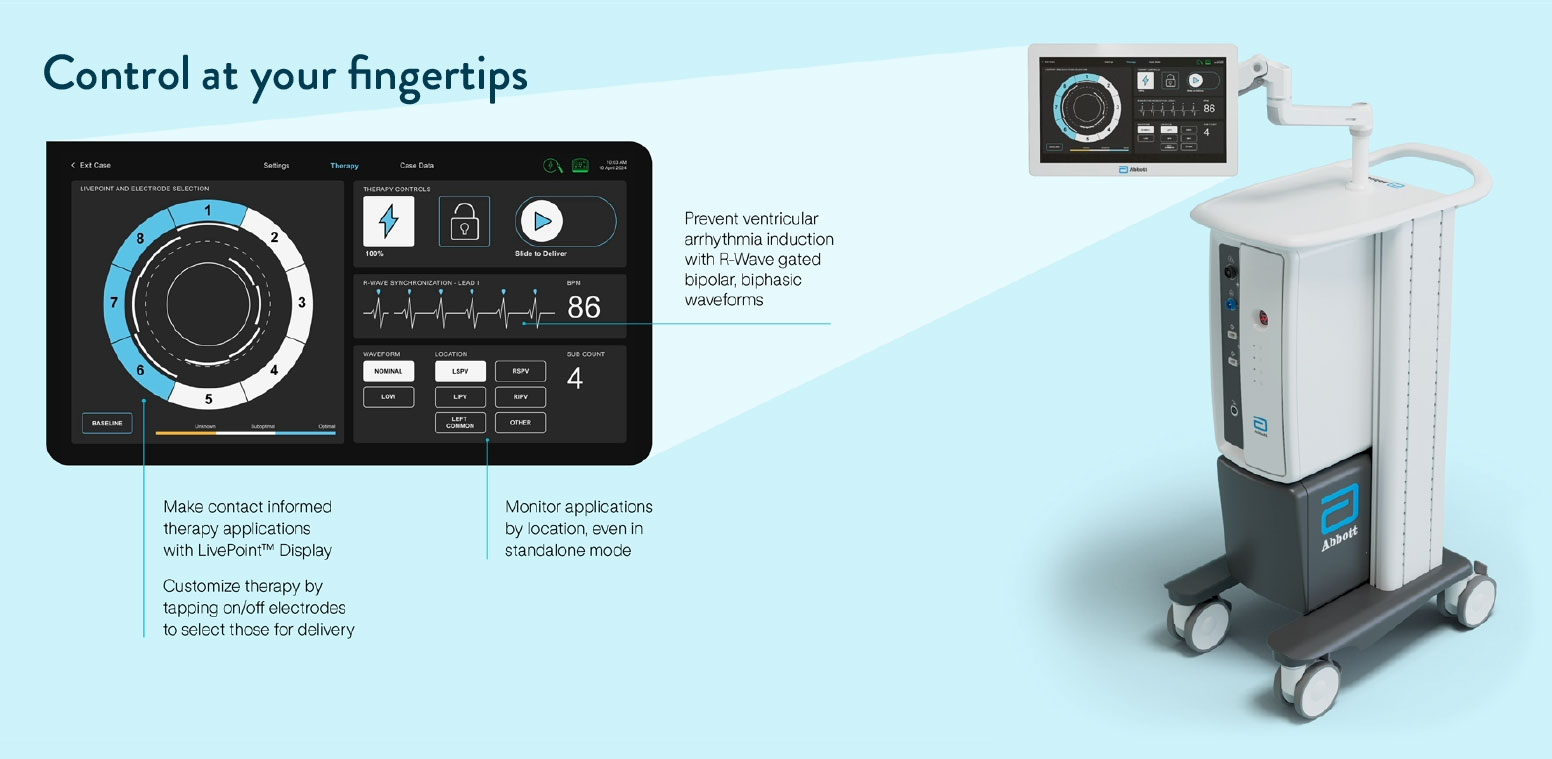
Ensures ease of use and adaptability with an intuitive generator – designed for now and the future
Current™ PFA Generator’s waveforms are designed to minimize microbubbles5 and patient movement for stability during energy delivery2
The only standalone generator with an integrated tissue contact display – empowering contact-guided energy applications, with or without a mapping system4
Features distal and proximal magnetic sensors for seamless EnSite™ X EP System integration
Eliminates the need for general anesthesia through light sedation compatibility6
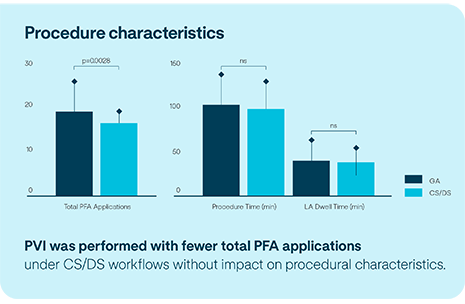
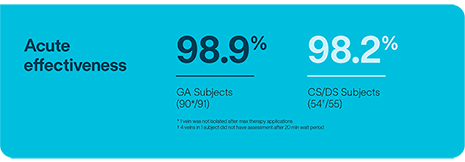
PVI with Volt™ PFA Catheter, Sensor Enabled™ in subjects under deep or conscious sedation (CS/DS) does not significantly impact safety or acute outcomes compared to procedures with general anesthesia (GA).6
Create PVI with as few as two applications per vein1
The lowest number of applications of any PFA tool1 minimizing catheter repositions and increasing procedural efficiency1
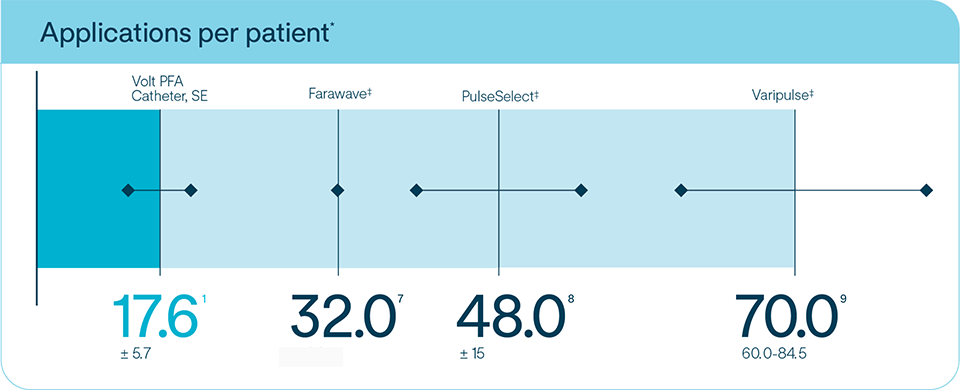
*Based on published data from multicenter experience and separate studies, which may involve different patient populations and other variables. Not a head-to-head comparison. Data presented for informational purposes only.
Customize therapy for anatomic and patient variation by selectively delivering energy from electrodes in good contact
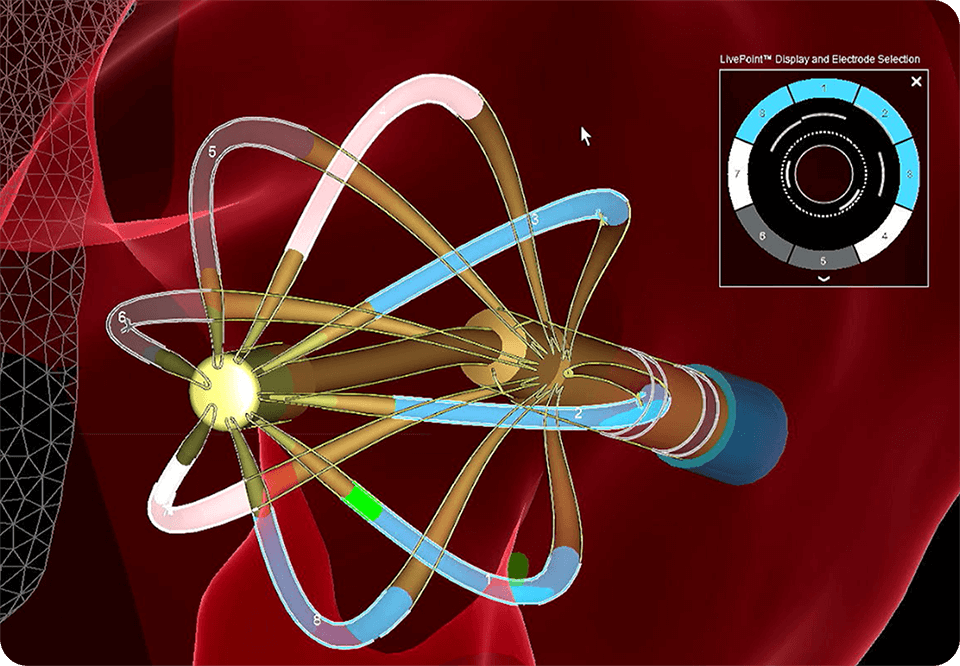
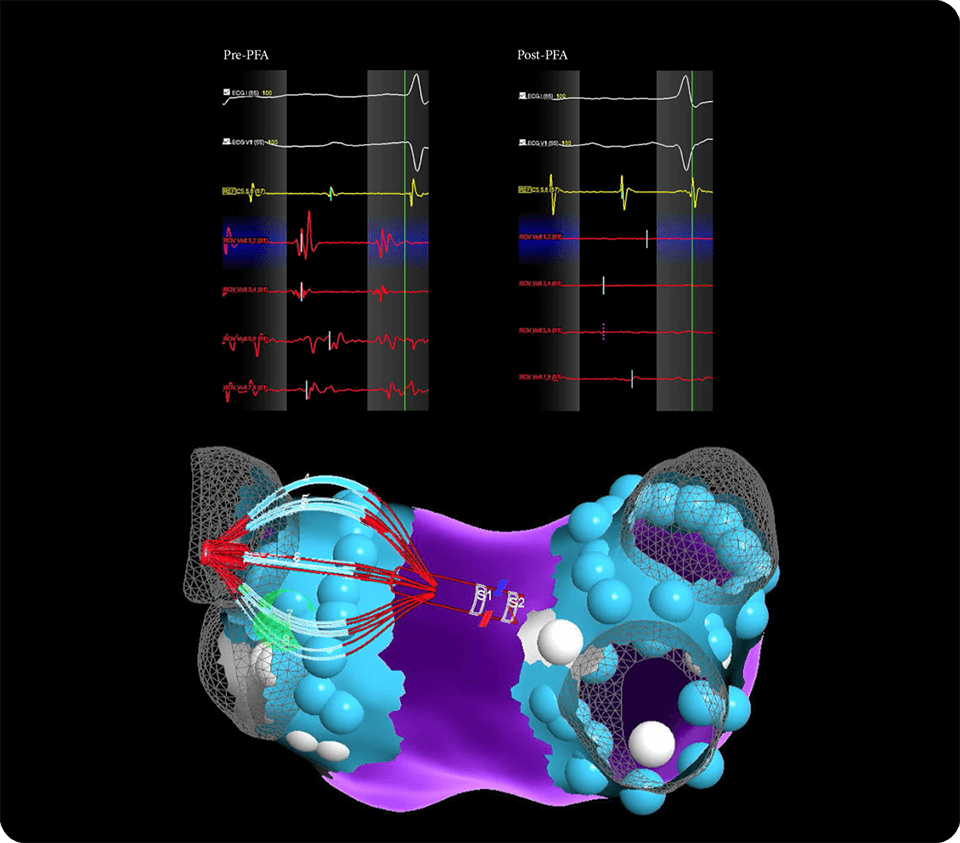
Enable a single-catheter workflow for mapping, pacing and ablating, minimizing catheter exchanges
Data from the Volt CE Mark study demonstrates that PVI with the Volt™ PFA System is both safe and effective for treating PAF and PsAF over a 6-month follow-up period.10
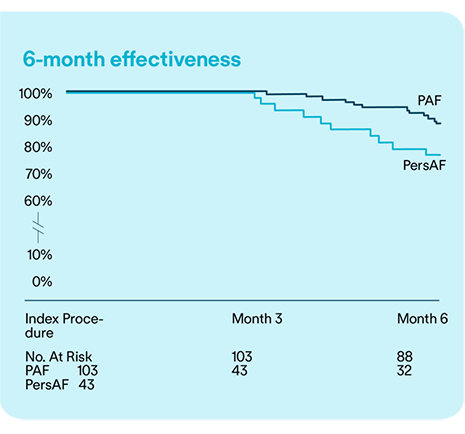
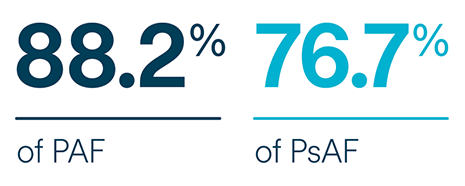
subjects free from documented AF/AFL/AT recurrence at 6-months
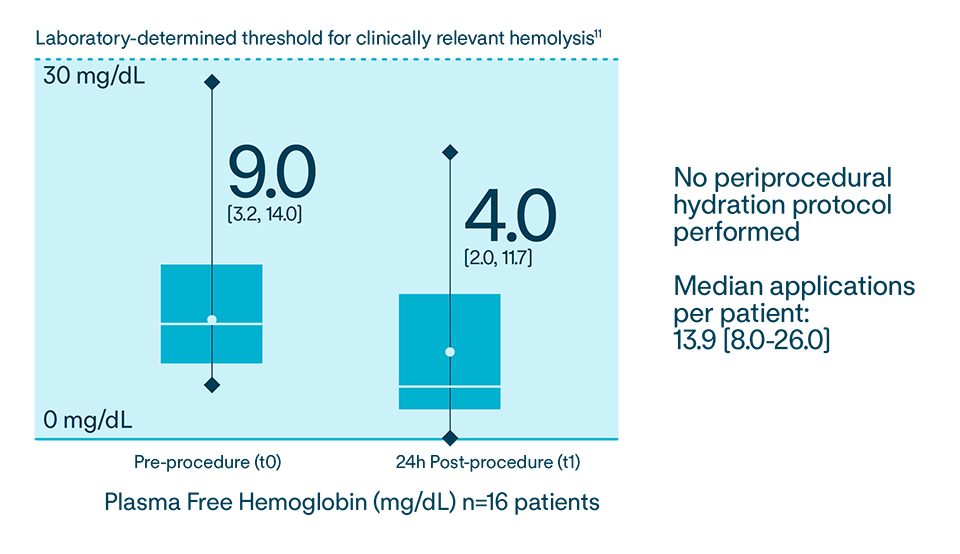
Reduces hemolysis11,12 by directly targeting tissue, avoiding the blood pool, and removing the need for additional fluid11


This device is commercially available for use in select international markets.
* Faster procedures allows us to treat more patients.
** In listed CE Mark or IDE trials for achievement of PVI.
*** Per protocol definition of isolation defined by entrance block after 20-minute wait period. 1 subject did not complete 20 minute wait period. 1 vein in 1 subject was not isolated after maximum allowed applications.
MAT-2502382 v2.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0