The market-leading Agilis NxT Steerable Introducer is now compatible with larger catheters to support all therapy deliveries from PFA to RF to CRYO - for an optimal workflow with our One System Solution. Introducing the Agilis NxT Steerable Introducer, Dual‑Reach™ (13 Fr).

ATRAUMATIC TIP
New low-profile design and atraumatic tip
SIX OFF SET VENTING HOLES
Smaller diameter (.030”) and 90º offset reduces the likelihood of guidewire exiting through side holes
RADIO-OPAQUE TIP MARKER
Ring of platinum iridium enables visualization under fluoro; 4 mm from tip
SHEATH TO DILATOR FIT
Low-profile design with lower transeptal crossing force
BRAIDED SHAFT
Provides exceptional torquability, pushability and kink resistance with Dual-Reach™ bidirectional 180˚/90˚ deflection to allow asymmetric deflection for more mobility
BI-DIRECTIONAL; ROTATING COLLAR
To deflect the tip clockwise ≥180° and counterclockwise ≥90° auto locking
HEMOSTASIS VALVE
3-way stopcock for air or blood aspiration, fluid infusion, blood sampling
As evidenced in more than one million procedures*, for a growing number of physicians, Agilis NxT Steerable Introducer (8.5 Fr) is the introducer of choice when guiding ablation catheters to treat arrhythmias. Tailor to your patient’s anatomy with a large variety of sizes and curves that maintain their shape
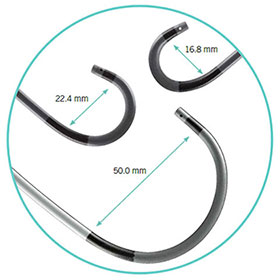
Curves shown is for 8.5 Fr Agilis NxT Steerable Introducer
Enhanced catheter stability enables control of tip movements within millimeters along the intended ablation line1
Access hard-to-reach areas with precise maneuverability and auto-lock steering, such as the right inferior and left anterior pulmonary veins1
Greater catheter-tissue contact force, which may support more durable lesion formation2
Greater freedom from arrhythmia at 6 months (76.0% vs 53.0%)3 and 18% greater at 12 months (74.0% vs 62.7%)4
Complete PVI isolation (left- and right-sided veins) (43 pts vs 8 pts)1
Fewer re-do ablations (6 pts vs 23 pts)1 and 17% fewer acute PV reconnections (50% vs 60%)5
*Based on sales through Dec 31, 2020
Information herein intended for distribution outside the U.S. only, excluding Finland.
MAT-2414395 v2.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0