The LIFE-BTK Study, published in the New England Journal of Medicine, is the first successful RCT to demonstrate superiority of investigational device over standard of care for treatment of BTK disease in CLTI patients1. The 2-year data presented at VIVA 2024 demonstrated sustained efficacy & safety2.

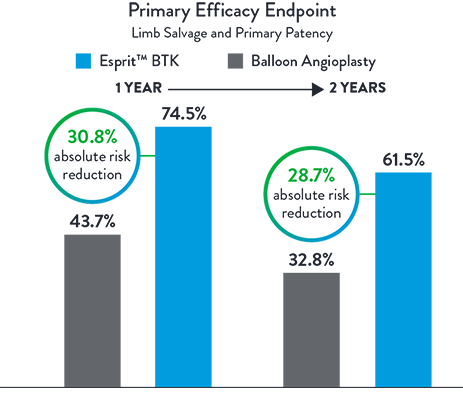
Esprit™ BTK offers continued superior long-term clinical outcomes vs PTA, particularly in terms of limb salvage and primary patency at 2 years. A clear advantage over PTA in terms of sustained vascular patency and limb preservation.2
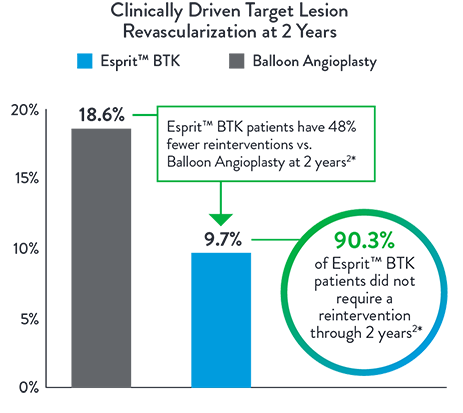
Esprit™ BTK significantly reduces binary restenosis and total occlusion, due to its biological efficacy and resorbable mechanical support. Esprit™ BTK demonstrates long-term durability, effectively reducing restenosis and promoting long-term vessel patency.
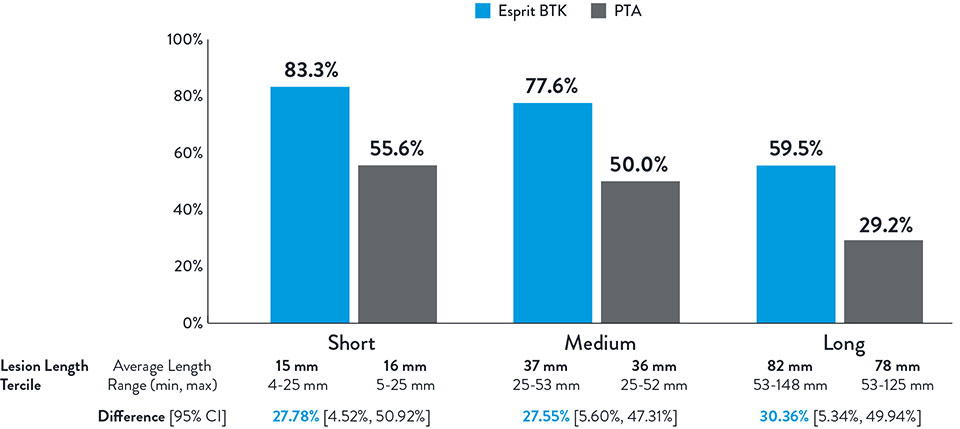
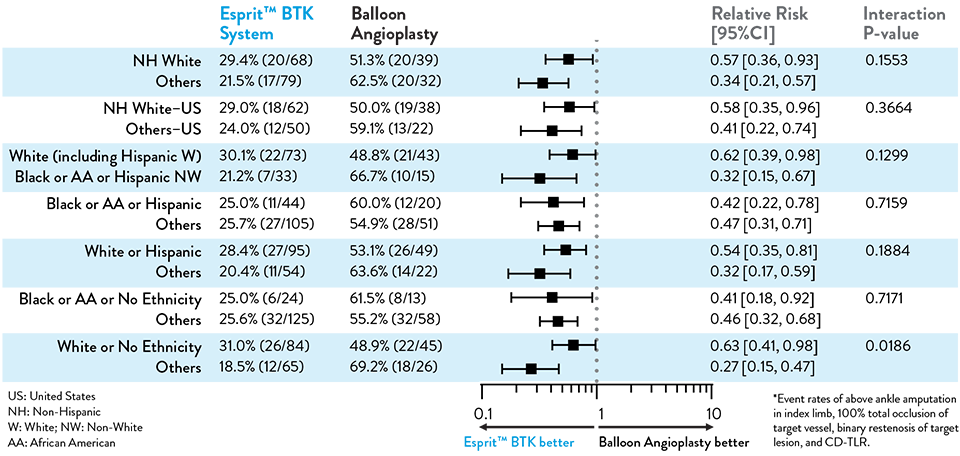
NOTE: Post-hoc subgroup analysis by lesion length terciles was conducted where no pre-specified hypothesis testing was completed to provide a p-value.
Varcoe, Ramon L., et. al. "Supplementary Appendix." In "Drug-eluting resorbable scaffold versus angioplasty for infrapopliteal artery disease." New England Journal of Medicine. 390 (2024): 9-19.
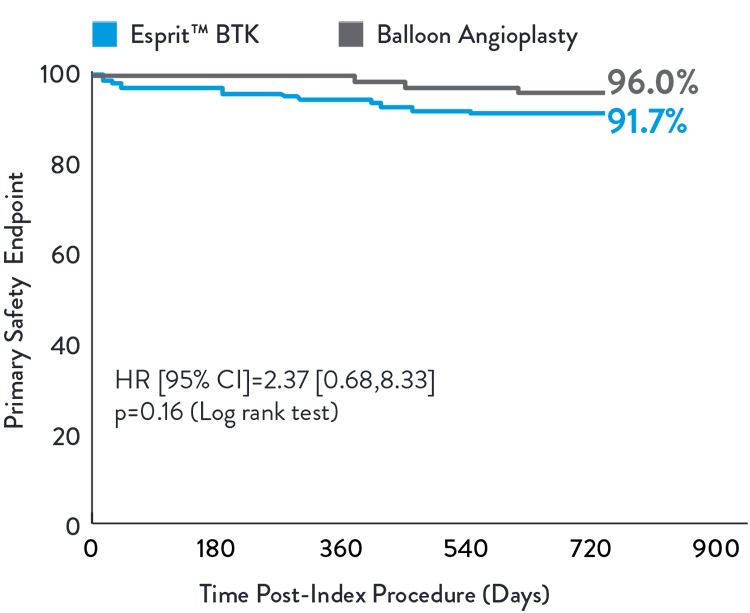
The LIFE-BTK Study is a prospective, multicenter, randomized controlled trial to evaluate the safety and efficacy of the Esprit™ BTK Everolimus Eluting Resorbable Scaffold System vs percutaneous transluminal angioplasty (PTA)† for the treatment of infrapopliteal arterial disease in patients with chronic limb-threatening ischemia (CLTI).
* clinicaltrials.gov/study/NCT04227899
** Follow up focused on index wound assessment
† defined as Percutaneous Transluminal Angioplasty
Varcoe, R. Primary Outcomes of the Esprit™ BTK Drug-Eluting Resorbable Scaffold for the Treatment of Infrapopliteal Lesions: The LIFE-BTK Trial. Presented at TCT 2023
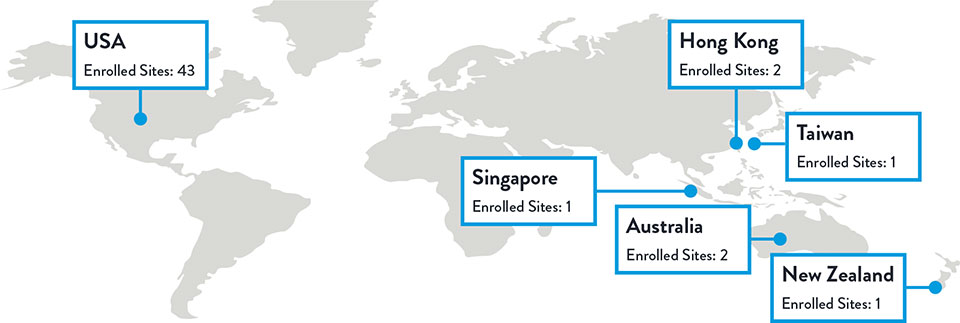
Multiple studies have shown racial and ethnic disparities in the prevalence of PAD and CLTI, as well as in access to and outcomes of treatment3,4
The LIFE-BTK trial was designed and conducted to include a diverse patient population representative of those most affected by the disease5
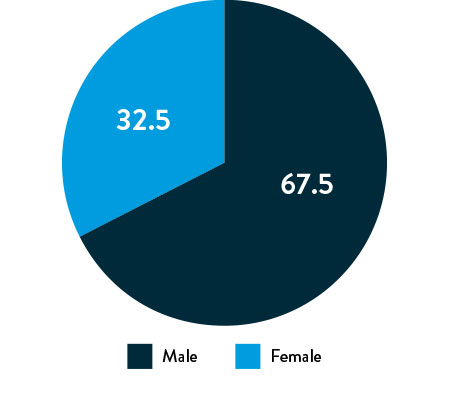
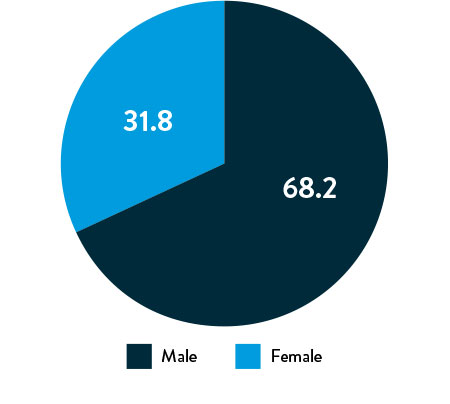
Race, Gender, and Ethnicity Distribution
Gender
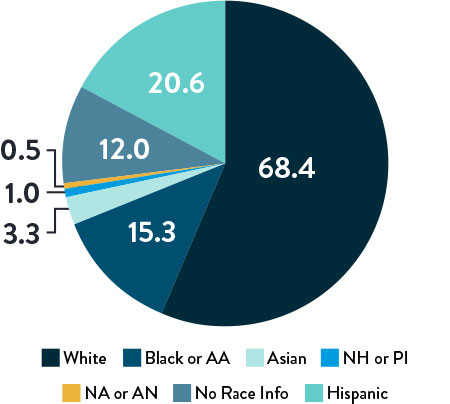
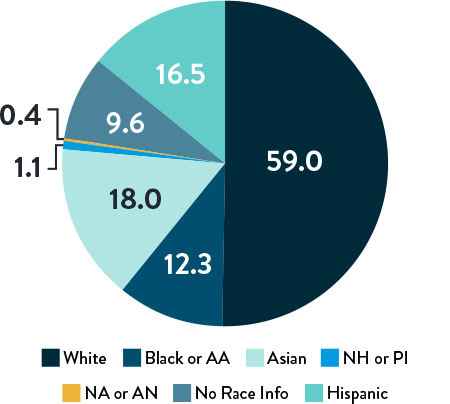
AA: African American | NH: Native Hawaiian | PI: Pacific Islander | NA: North American Native/American Indian | AN: Alaska Native
Varcoe, R. Primary Outcomes of the Esprit™ BTK Drug-Eluting Resorbable Scaffold for the Treatment of Infrapopliteal Lesions: The LIFE-BTK Trial. Presented at TCT 2023
The primary and secondary endpoint results for different race and ethnicity subgroups were consistent with the overall population, demonstrating the Esprit™ BTK device’s biological efficacy, durability, and robust performance across diverse patient populations5.
| PRIMARY EFFICACY ENDPOINT | PRIMARY SAFETY ENDPOINT | |
|---|---|---|
| Endpoint | Limb Salvage + Primary Patency | Freedom from MALE + POD |
| Definition | Freedom from above ankle amputation in index limb, 100% total occlusion of target vessel, binary restenosis of target lesion, and CD-TLR* at 12 months | MALE = Above ankle amputation in index limb, major re-intervention at 6 monthsPOD = Perioperative mortality at 30 days |
| Test | Superiority of Esprit™ BTK against PTA with a 1-sided a of 0.0249 | Non-inferiority of Esprit™ BTK against PTA with a 1-sided a of 0.025. |
| 1st SECONDARY ENDPOINT | 2nd SECONDARY ENDPOINT | |
|---|---|---|
| Endpoint | Binary restenosis of the target lesion at 1 year | Freedom from above ankle amputation in index limb, 100% total occlusion of target vessel and CD-TLR at 1 year |
| Test | Superiority of Esprit™ BTK against PTA with a 1-sided a of 0.025 | Superiority of Esprit™ BTK against PTA with a 1-sided a of 0.025. |
| Lesion Characteristics | Esprit BTK | PTA |
|---|---|---|
| Lesion length (mm) | 43.78 ± 31.84 | 44.75 ± 29.07 |
| RVD pre-intervention (mm) | 2.94 ± 0.77 | 2.82 ± 0.74 |
| Site-Reported Calcification (Moderate/Severe) | 30.8% | 30.5% |
| %DS pre-intervention | 72.6 ± 18.9 | 73.7 ± 21.0 |
| Patient Demographics* | Esprit BTK | PTA |
|---|---|---|
| Hypertension | 94.2% | 90.9% |
| Hyperlipidemia | 80.9% | 81.8% |
| Tobacco Use | 52.6% | 53.4% |
| Diabetes | 71.7% | 69.3% |
| Rutherford Becker 4 | 52.0% | 51.1% |
| Rutherford Becker 5 | 48.0% | 48.9% |
| Prior PAD | Esprit 82.7% | PTA 77.3% |
*All patients in the LIFE-BTK trial presented with CLTI with either ischemic rest pain (Rutherford-Becker class 4) or minor tissue loss (Rutherford-Becker class 5) along with multiple risk factors.
Resorbable scaffold backbone comprised of 100% poly (L-lactide) (PLLA) and strut thickness of 99µm**

Coating comprised of the active pharmaceutical ingredient everolimus and resorbable poly (D,L- lactide) (PDLLA)

Four platinum markers of the same mass, two each embedded at the proximal and distal ends of the scaffold for radiopacity***

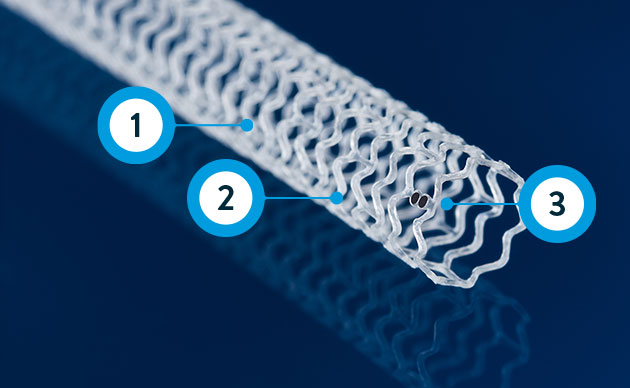
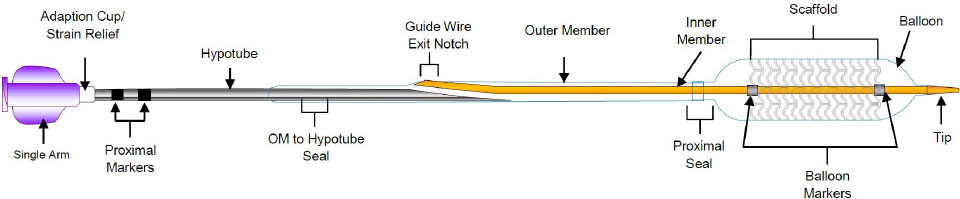
** ≤ 3.0 mm size; 3.5-3.75 mm sizes have 120 µm strut thickness.
***Platinum markers at proximal and distal ends remain for angiographic visualization
Data on file at Abbott.
Photos on file at Abbott.
Abbott continues to study Esprit BTK in real-world patient populations. Learn more about the Esprit BTK Post-Approval Study.
References:
†Superiority testing performed at 1 year.
*Re-intervention defined as CD-TLR.
MAT-2403292 v3.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0