The LIFE-BTK Study, published in the New England Journal of Medicine, is the first successful RCT to demonstrate superiority of investigational device over standard of care for treatment of BTK disease in CLTI patients1. The 2-year data presented at VIVA 2024 demonstrated sustained efficacy & safety2.
Clinical Follow-Up

LIFE-BTK Study Results
Superior Efficacy, Sustained Benefits through 2 Years2†
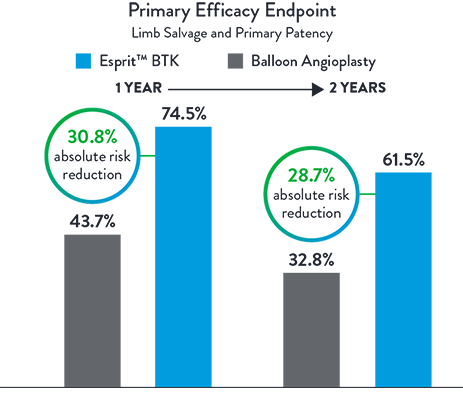
Esprit™ BTK offers continued superior long-term clinical outcomes vs PTA, particularly in terms of limb salvage and primary patency at 2 years. A clear advantage over PTA in terms of sustained vascular patency and limb preservation.2
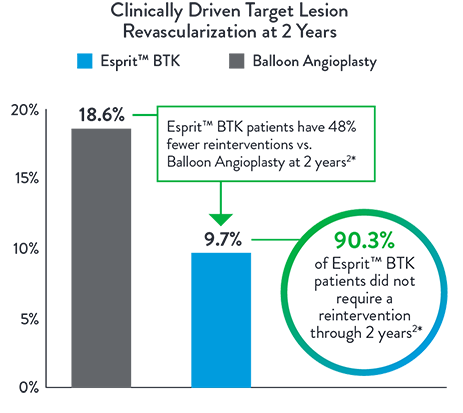
48% Fewer Patients Require Re-Intervention at 2 Years2*
Esprit™ BTK significantly reduces binary restenosis and total occlusion, due to its biological efficacy and resorbable mechanical support. Esprit™ BTK demonstrates long-term durability, effectively reducing restenosis and promoting long-term vessel patency.
Consistently Higher Observed Rate of Limb Salvage and Primary Patency With
Esprit™ BTK Across a Range of Lesion Lengths Up to 148 mm
Primary Efficacy Endpoint
Composite of Limb Salvage and Primary Patency at 1 Year
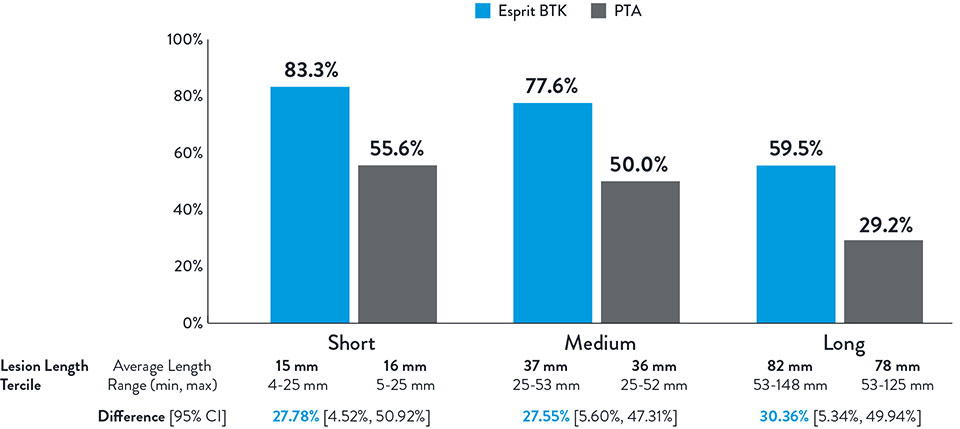
NOTE: Post-hoc subgroup analysis by lesion length terciles was conducted where no pre-specified hypothesis testing was completed to provide a p-value.
Varcoe, Ramon L., et. al. "Supplementary Appendix." In "Drug-eluting resorbable scaffold versus angioplasty for infrapopliteal artery disease." New England Journal of Medicine. 390 (2024): 9-19.
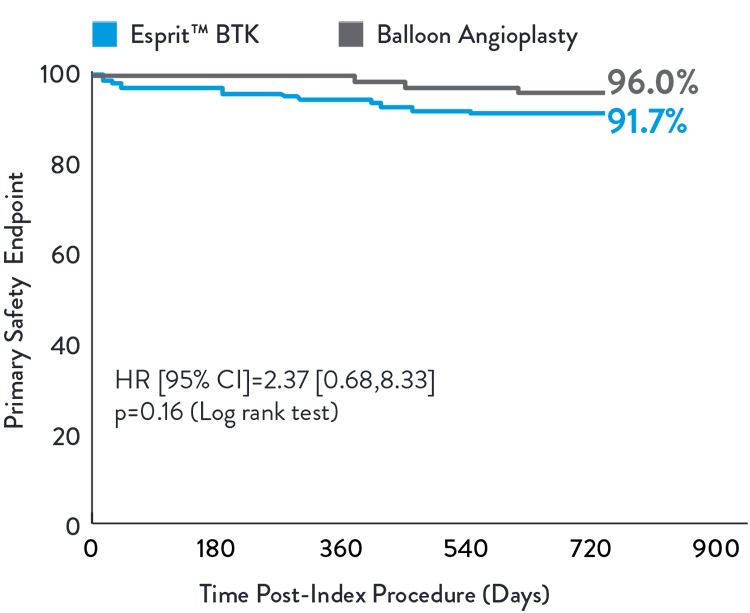
Esprit™ BTK demonstrated to be as safe as balloon angioplasty, maintained at 2 years.2
Composite of MALE (2-year) and POD (30-day)
LIFE-BTK Study Design*
The LIFE-BTK Study is a prospective, multicenter, randomized controlled trial to evaluate the safety and efficacy of the Esprit™ BTK Everolimus Eluting Resorbable Scaffold System vs percutaneous transluminal angioplasty (PTA)† for the treatment of infrapopliteal arterial disease in patients with chronic limb-threatening ischemia (CLTI).
- Prospective, randomized, multicenter trial across 50 Global Sites
- 261 patients randomized (2:1 Esprit™ BTK System vs. PTA†)
- Esprit™ BTK System (n=173)
- PTA† (n=88)
* clinicaltrials.gov/study/NCT04227899
** Follow up focused on index wound assessment
† defined as Percutaneous Transluminal Angioplasty
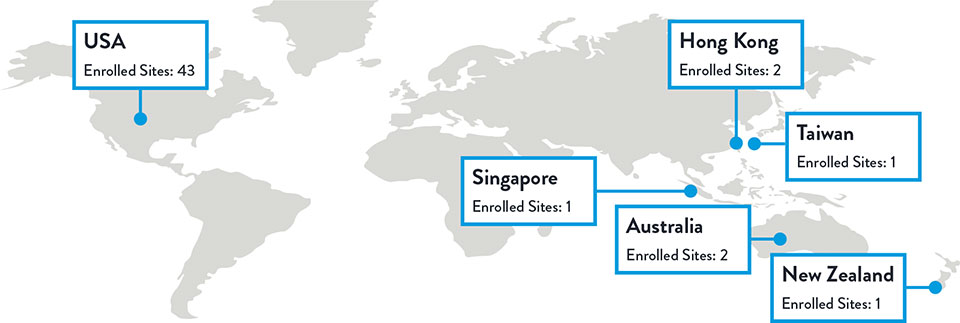
Sites and Enrollment
Total Enrolled Sites: 50 | Total Randomized Patients: 261
Varcoe, R. Primary Outcomes of the Esprit™ BTK Drug-Eluting Resorbable Scaffold for the Treatment of Infrapopliteal Lesions: The LIFE-BTK Trial. Presented at TCT 2023
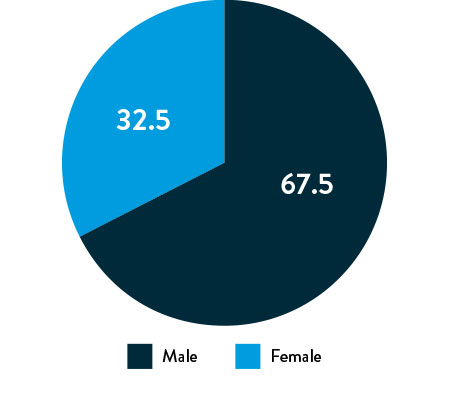
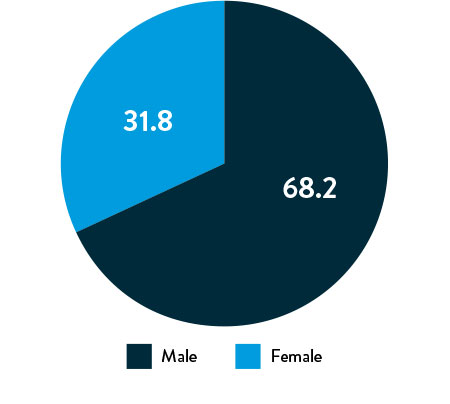
Race, Gender, and Ethnicity Distribution
Multiple studies have shown racial and ethnic disparities in the prevalence of PAD and CLTI, as well as in access to and outcomes of treatment3,4
The LIFE-BTK trial was designed and conducted to include a diverse patient population representative of those most affected by the disease5
Race, Gender, and Ethnicity Distribution
Gender
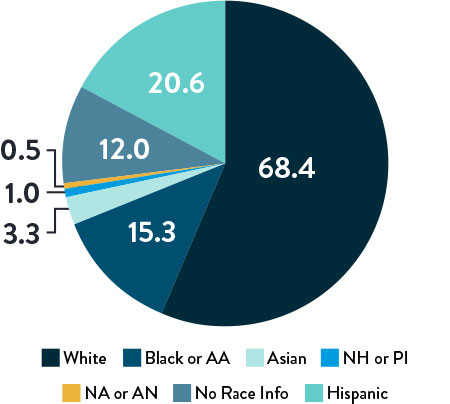
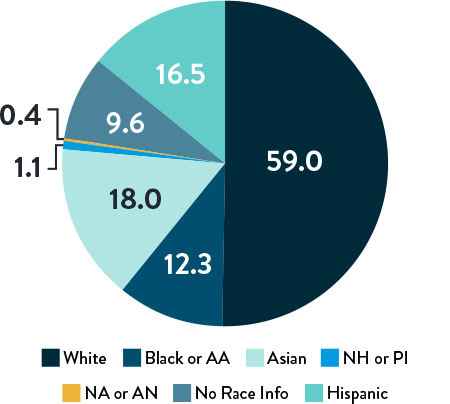
Race and Ethnicity
AA: African American | NH: Native Hawaiian | PI: Pacific Islander | NA: North American Native/American Indian | AN: Alaska Native
Varcoe, R. Primary Outcomes of the Esprit™ BTK Drug-Eluting Resorbable Scaffold for the Treatment of Infrapopliteal Lesions: The LIFE-BTK Trial. Presented at TCT 2023
Proven Efficacy and Durability Across Diverse Races and Ethnicities
Composite Primary Effectiveness Endpoint at 1 Year
The primary and secondary endpoint results for different race and ethnicity subgroups were consistent with the overall population, demonstrating the Esprit™ BTK device’s biological efficacy, durability, and robust performance across diverse patient populations5.
Race and Ethnicity Subgroup Analysis
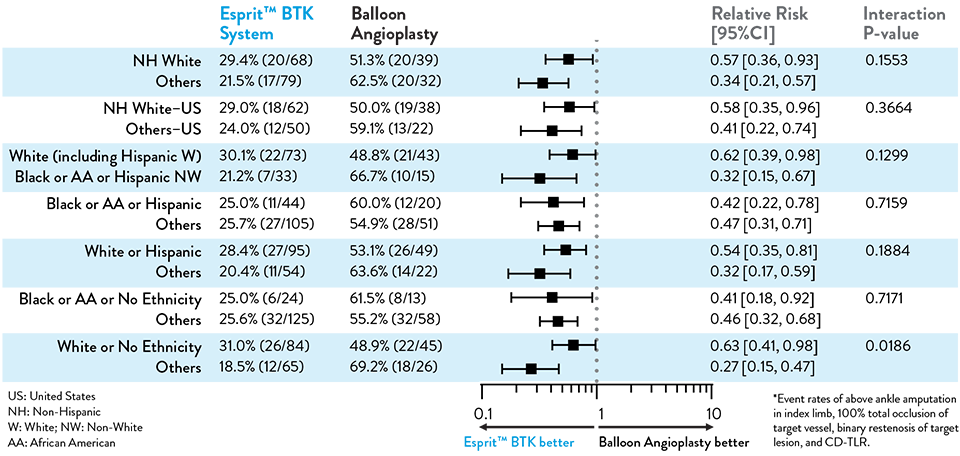
LIFE-BTK 1Y Endpoints
| PRIMARY EFFICACY ENDPOINT | PRIMARY SAFETY ENDPOINT | |
|---|---|---|
| Endpoint | Limb Salvage + Primary Patency | Freedom from MALE + POD |
| Definition | Freedom from above ankle amputation in index limb, 100% total occlusion of target vessel, binary restenosis of target lesion, and CD-TLR* at 12 months | MALE = Above ankle amputation in index limb, major re-intervention at 6 monthsPOD = Perioperative mortality at 30 days |
| Test | Superiority of Esprit™ BTK against PTA with a 1-sided a of 0.0249 | Non-inferiority of Esprit™ BTK against PTA with a 1-sided a of 0.025. |
| 1st SECONDARY ENDPOINT | 2nd SECONDARY ENDPOINT | |
|---|---|---|
| Endpoint | Binary restenosis of the target lesion at 1 year | Freedom from above ankle amputation in index limb, 100% total occlusion of target vessel and CD-TLR at 1 year |
| Test | Superiority of Esprit™ BTK against PTA with a 1-sided a of 0.025 | Superiority of Esprit™ BTK against PTA with a 1-sided a of 0.025. |
Implanted Vessel Sites
Baseline Lesion & Patient Characteristics
| Lesion Characteristics | Esprit BTK | PTA |
|---|---|---|
| Lesion length (mm) | 43.78 ± 31.84 | 44.75 ± 29.07 |
| RVD pre-intervention (mm) | 2.94 ± 0.77 | 2.82 ± 0.74 |
| Site-Reported Calcification (Moderate/Severe) | 30.8% | 30.5% |
| %DS pre-intervention | 72.6 ± 18.9 | 73.7 ± 21.0 |
| Patient Demographics* | Esprit BTK | PTA |
|---|---|---|
| Hypertension | 94.2% | 90.9% |
| Hyperlipidemia | 80.9% | 81.8% |
| Tobacco Use | 52.6% | 53.4% |
| Diabetes | 71.7% | 69.3% |
| Rutherford Becker 4 | 52.0% | 51.1% |
| Rutherford Becker 5 | 48.0% | 48.9% |
| Prior PAD | Esprit 82.7% | PTA 77.3% |
*All patients in the LIFE-BTK trial presented with CLTI with either ischemic rest pain (Rutherford-Becker class 4) or minor tissue loss (Rutherford-Becker class 5) along with multiple risk factors.
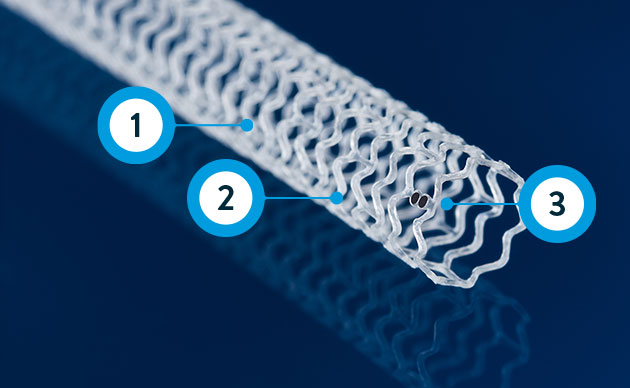
Esprit™ BTK System
Resorbable scaffold backbone comprised of 100% poly (L-lactide) (PLLA) and strut thickness of 99µm**

Coating comprised of the active pharmaceutical ingredient everolimus and resorbable poly (D,L- lactide) (PDLLA)

Four platinum markers of the same mass, two each embedded at the proximal and distal ends of the scaffold for radiopacity***

Delivery System
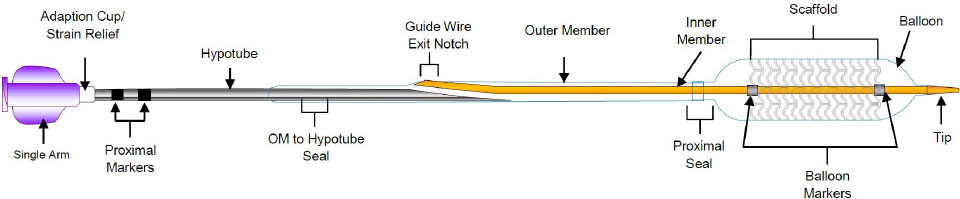
** ≤ 3.0 mm size; 3.5-3.75 mm sizes have 120 µm strut thickness.
***Platinum markers at proximal and distal ends remain for angiographic visualization
Data on file at Abbott.
Photos on file at Abbott.
Abbott continues to study Esprit BTK in real-world patient populations. Learn more about the Esprit BTK Post-Approval Study.
References:
†Superiority testing performed at 1 year.
*Re-intervention defined as CD-TLR.
- Varcoe, RL., et al. Drug-Eluting Resorbable Scaffold versus Angioplasty for Infrapopliteal Artery Disease. N Eng J Med 2024;390:9-19.
- Brian G. DeRubertis et al., Two-Year Outcomes of the LIFE-BTK Randomized Controlled Trial Evaluating the Esprit™ BTK Drug-eluting Resorbable Scaffold for Treatment of Infrapopliteal Lesions, VIVA 2024.
- Eid MA, et al. Semin Vasc Surg. 2021;34(1):38-46.
- Chen L, et al. Front Cardiovasc Med. 2021;8:692236.
- Garcia, LA, et al. Diversity, Equity, and Inclusion in the LIFE-BTK Trial Evaluating the Esprit™ BTK Drug-Eluting Resorbable Scaffold for the Treatment of Infrapopliteal Lesions in Patients with Chronic Limb-Threatening Ischemia, VIVA 2024.
MAT-2401524 v3.0
Important Safety Information
Esprit™ BTK Everolimus Eluting Resorbable Scaffold System

Indications
The Esprit™ BTK Everolimus Eluting Resorbable Scaffold System is indicated for improving luminal diameter in infrapopliteal lesions in patients with chronic limb-threatening ischemia (CLTI) and total scaffolding length up to 170 mm with a reference vessel diameter of ≥ 2.5 mm and ≤ 4.00 mm.
Contraindications
The Esprit™ BTK Everolimus Eluting Resorbable Scaffold System is contraindicated for use in:
- Patients who cannot tolerate, including allergy or hypersensitivity to, procedural anticoagulation or the post-procedural antiplatelet regimen.
- Patients with hypersensitivity or contraindication to everolimus or structurally related compounds or known hypersensitivity to scaffold components poly(L-lactide), poly(D, L-lactide), and platinum.
Warnings
- This device is intended for single use only. Do not reuse, reprocess, or re-sterilize. Note the product "Use-by" date on the package. Reuse, reprocessing, or re-sterilization may compromise the structural integrity of the device and / or delivery system and / or lead to device failure, which may result in patient injury, illness, or death. Reuse, reprocessing, or re-sterilization may also create a risk of contamination of the device and / or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device and / or delivery system may lead to injury, illness, or death of the patient.
- The Esprit™ BTK System is intended to perform as a system. The scaffold should not be removed for use with other dilatation catheters.
- The Esprit™ BTK System should not be used in conjunction with other non-everolimus drug eluting devices in the same vessel as the Esprit™ BTK Scaffold.
- It is not recommended to use this scaffold to treat lesions located at any joint or other hinge points, such as the knee or ankle. The recommended region for below-the-knee (BTK) treatment with the Esprit™ BTK Scaffold is the infrapopliteal arteries at a location ≥ 10 cm above the proximal margin of the ankle mortise. The Esprit™ BTK Scaffold has not been tested for use outside the recommended implant locations.
- This product should not be used in patients with aneurysms immediately adjacent to the scaffold implantation site.
- Insertion of the Esprit™ BTK System and implantation of the scaffold should be performed only under fluoroscopic observation with radiographic equipment providing high resolution images.
- Quantitative imaging is strongly recommended to accurately measure and confirm appropriate vessel sizing (reference vessel diameter ≥ 2.5 mm). If quantitative imaging determines a vessel size < 2.5 mm, do not implant the Esprit™ BTK scaffold.
- Adequate lesion preparation prior to scaffold implantation is required to ensure safe delivery of the scaffold across the target lesion. It is not recommended to treat patients having a lesion that prevents complete inflation of an angioplasty balloon.
- Successful pre-dilatation with residual diameter stenosis of < 30% by visual estimation is required for treatment of the target lesion; < 20% by visual estimation is preferred.
- Ensure the scaffold is not post-dilated beyond the allowable expansion limits.
- Use of appropriate anticoagulant and / or antiplatelet therapy per standard of care is recommended for use of this scaffold system.
- This product should not be used in patients who are not likely to comply with the recommended antiplatelet therapy.
- Judicious selection of patients is necessary, since the use of this device carries the associated risk of scaffold thrombosis, vascular complications, and / or bleeding events.
Precautions
- Scaffold placement should not be performed in patients with known allergies to contrast agent that cannot be medically managed.
- It is not recommended to treat patients having a lesion with excessive tortuosity proximal to or within the lesion.
- When multiple scaffolds are required, only combinations of Esprit™ BTK Scaffolds must be used. Any potential interaction with other drug-eluting or coated devices has not been evaluated.
- The delivery system is intended for deployment of the scaffold only and should not be used to dilate other locations.
- Implantation of the scaffold should be performed only by physicians who have received appropriate training.
- As with all catheter-based procedures, scaffold placement should be performed at facilities where patient can be prepared for necessary intervention and / or surgical removal of the device and vessel repair as per facility protocol.
- Pre-dilatation should be performed with an angioplasty balloon. Cutting or scoring balloons can be used per physician discretion, if the lesion appears to be mildly calcified.
- Failure to pre-dilate the vessel may impair nominal / optimal scaffold delivery.
- Implanting a scaffold may lead to dissection of the vessel distal and / or proximal to the scaffold, requiring additional intervention.
Note: In cases of bailouts, bailout treatment of the target lesion can be done using the Esprit™ BTK Scaffold of the appropriate length. If an appropriate length Esprit™ BTK Scaffold is not available, physicians should use standard of care. - An unexpanded scaffold may be retracted into the introducer sheath one time only. An unexpanded scaffold should not be reintroduced into the artery once it has been pulled back into the introducer sheath.
- Post-dilatation is strongly recommended for optimal scaffold apposition. When performed, post-dilatation should be performed at high pressure (> 16 atm) with a non-compliant balloon up to 0.5 mm larger than the nominal scaffold diameter.
- Use an appropriately sized non-drug coated balloon to pre-dilate the lesion. When treating a long lesion, scaffold the distal portion of the lesion prior to scaffolding the proximal portion of the lesion.
- Ensure that the scaffolded area covers the entire lesion / dissection site and that no gaps exist between scaffolds.
- The extent of the patient’s exposure to drug and polymer is directly related to the number of scaffolds implanted. The safety of everolimus, polymer, and polymer breakdown products was evaluated in pre-clinical studies and the biocompatibility assessment of the Esprit™ BTK Scaffold.
- The safety and effectiveness of the Esprit™ BTK Scaffold in patients with prior brachytherapy of the target lesion or the use of brachytherapy for treated-site restenosis in the Esprit™ BTK Scaffold have not been established. Both vascular brachytherapy and the Esprit™ BTK Scaffold alter arterial modeling. The potential combined effect on arterial remodeling by these two treatments is not known.
- The safety and effectiveness of the Esprit™ BTK System have not been established in clinical trials with the use of either mechanical atherectomy devices (directional atherectomy catheters, rotational atherectomy catheters) or laser atherectomy catheters.
- Formal drug interaction studies have not been performed with the Esprit™ BTK Scaffold because of limited exposure to everolimus eluted from the scaffold.
- Everolimus, the Esprit™ BTK Scaffold’s active pharmaceutical ingredient, is an immunosuppressive agent. Therefore, consideration should be given to patients taking other immunosuppressive agents or who are at risk for immune suppression.
- Oral everolimus use in renal transplant and advanced renal cell carcinoma patients was associated with increased serum cholesterol and triglyceride levels, which in some cases required treatment.
- Non-clinical testing has demonstrated the Esprit™ BTK Scaffold is MR Conditional. A person with the Esprit™ BTK Scaffold may be safely scanned under the following conditions. Failure to follow these conditions may result in injury
- Static magnetic field strength of 7 Tesla or less
- The Esprit™ BTK Scaffold should not migrate in this MRI environment. MRI at 7 Tesla or less may be performed immediately following the implantation of the Esprit™ BTK Scaffold.
Potential Adverse Events
Potential adverse events include, but are not limited to:
Allergic reaction or hypersensitivity to contrast agent, anesthesia, scaffold materials (poly[L-lactide] [PLLA], poly[D, L-lactide] [PDLLA], platinum, or everolimus), and drug reactions to anticoagulation or antiplatelet drugs
- Vascular access complications which may require transfusion or vessel repair, including:
- Catheter site reactions
- Bleeding (ecchymosis, oozing, hematoma, hemorrhage, retroperitoneal hemorrhage)
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation / rupture, and laceration
- Embolism (air, tissue, plaque, thrombotic material, or device)
- Peripheral ischemia
- Target artery complications which may require additional intervention, including:
- Total occlusion or abrupt closure
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation / rupture
- Embolism (air, tissue, plaque, thrombotic material, or device)
- Artery or scaffold thrombosis
- Stenosis or restenosis
- Vasospasm
- Tissue prolapse / plaque shift
- Bleeding (non-access site)
- Additional surgery such as peripheral artery bypass graft surgery or amputation
- Peripheral nerve injury, neuropathy
- Compartment syndrome
- Tissue necrosis, gangrene, ulcer and acute limb ischemia
- Reperfusion injury
- New or worsening pain
- Intervention due to
- Damaged scaffolds
- Partial scaffold deployment
- Scaffold migration / unintentional placement of scaffold
- Other general surgical risks, including:
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias, and blocks)
- Stroke / cerebrovascular accident (CVA) and transient ischemic attack (TIA)
- Venous thromboembolism (including pulmonary embolism)
- Nausea and vomiting
- Hypotension / hypertension
- Infection – local and systemic (including post-procedural)
- Fever
- Blood cell disorders including heparin induced thrombocytopenia (HIT) and other coagulopathy
- Death
- System organ failures:
- Cardiac Failure
- Cardio-respiratory arrest (including pulmonary edema)
- Respiratory failure
- Renal failure
- Shock
The risks described below include the anticipated adverse events referenced in the contraindications, warnings, and precautions sections of the everolimus labels / SmPCs and / or observed at incidences ≥ 10% in clinical trials with oral everolimus for different indications. Refer to the drug SmPCs and labels for more detailed information and less frequent adverse events.
- Abdominal pain
- Anemia
- Angioedema (increased risk with concomitant angiotensin converting enzyme [ACE] inhibitor use)
- Arterial thrombotic events
- Bleeding and coagulopathy (including hemolytic uremic syndrome [HUS], thrombotic thrombocytopenic purpura [TTP], and thrombotic microangiopathy; increased risk with concomitant cyclosporine use)
- Constipation
- Cough
- Diabetes mellitus
- Diarrhea
- Dyspnea
- Embryo-fetal toxicity
- Erythema
- Erythroderma
- Headache
- Hepatic artery thrombosis (HAT)
- Hepatic disorders (including hepatitis and jaundice)
- Hypersensitivity to everolimus active substance, or to other rapamycin derivates
- Hypertension
- Infections (bacterial, viral, fungal, or protozoan infections, including infections with opportunistic pathogens). Polyoma virus-associated nephropathy (PVAN), JC virus associated progressive multiple leukoencephalopathy (PML), fatal infections and sepsis have been reported in patients treated with oral everolimus.
- Kidney arterial and venous thrombosis
- Laboratory test alterations (elevations of serum creatinine, proteinuria, hypokalemia, hyperkalemia; hyperglycemia, dyslipidemia including hypercholesterolemia and hypertriglyceridemia; abnormal liver function tests; decreases in hemoglobin, lymphocytes, neutrophils, and platelets)
- Lymphoma and skin cancer
- Male infertility
- Menstrual irregularities
- Nausea
- Nephrotoxicity (in combination with cyclosporine)
- Non-infectious pneumonitis (including interstitial lung disease)
- Oral ulcerations
- Pain
- Pancreatitis
- Pericardial effusion
- Peripheral edema
- Pleural effusion
- Pneumonia
- Pyrexia
- Rash
- Renal failure
- Upper respiratory tract infection
- Urinary tract infection
- Venous thromboembolism
- Vomiting
- Wound healing complications (including wound infections and lymphocele)
There may be other potential adverse events that are unforeseen at this time.
MAT-2403616 v1.0

Stay Connected