Unmatched Innovation: Setting the Pace with FDA-Approved AVEIR™ AR Atrial Leadless Pacemaker
Abbott Cardiac Rhythm Management | March 28, 2024
Introducing the world’s only atrial leadless pacemaker (LP), AVEIR AR LP is a revolutionary advancement in cardiac pacing technology that received FDA approval in June 20231. The device provides AAI(R) leadless pacing for your patients with sinus node dysfunction and normal AV and intraventricular conduction systems. This FDA approval signifies a significant milestone in the field of cardiology, expanding access to innovative leadless pacing solutions that eliminate the risks of lead- and pocket-related complications associated with traditional pacemakers and offer no visible scarring, pacemaker bulge, or arm movement restrictions.
The groundbreaking technology behind AVEIR AR LP aligns with the FDA's commitment to enhancing patient safety and accessibility to cutting-edge medical devices. Clinical data from a New England Journal of Medicine study supports the efficacy and safety of AVEIR™ AR LP. Early clinical evidence demonstrated a 95.2% electrical performance success rate in patients with acceptable atrial device capture threshold and sensing amplitude* while 90.3% of patients remained free from complications within 90 days, surpassing the study’s set performance goal of 78%.**2
Additionally, AVEIR AR LP is designed to accommodate the size and sensitivity of the right atrium, with unique features to achieve implant stability and optimization. A 1.63 mm inactive outer helix provides primary fixation while the recessed inner helix acts as the pacing electrode and provides additional fixation and electrical stability. The mapping prior to fixation capability ensures optimal electrical measurements in the atrium prior to device fixation, further enhancing patient safety.1,3
Engineered with upgradeability in mind, AVEIR AR LP can be paired with a ventricular leadless pacemaker to achieve dual chamber pacing.3 The AVEIR™ DR Dual Chamber LP System, comprising the AVEIR™ VR single chamber device and the newly approved AVEIR AR single chamber device, incorporates Abbott's novel implant-to-implant (i2i™) technology. This technology enables beat-to-beat communication between the two leadless pacemakers, facilitating dual chamber, leadless synchronous pacing.1,2,3 By ensuring continuous AV synchrony, regardless of patient posture, the AVEIR DR LP System offers an excellent alternative pacing solution for all your patients with abnormal or slow heart rhythms who require a pacemaker.
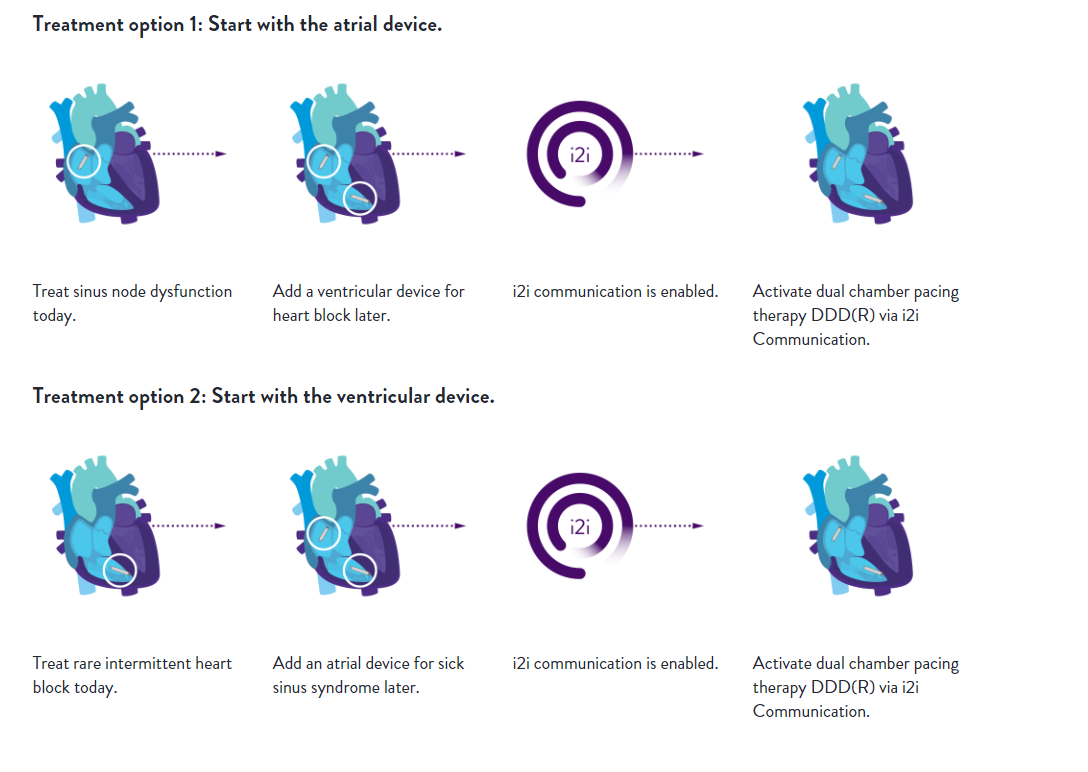
With its innovative features and promising clinical evidence, AVEIR AR LP is poised to revolutionize leadless pacing, providing your patients across the U.S. with access to advanced medical care and no visible or physical reminder of a pacemaker under the skin and fewer lead-related complications compared to transvenous pacemakers4,5. Learn more about leadless pacing solutions here.
* Atrial device capture threshold of ≤3.0 V @ 0.4 ms and ≥0.5 mV at 3-month visit.
** The inclusion of arrhythmias as a safety endpoint increased the overall incidence of complications as compared with other studies of leadless pacemakers, which excluded arrhythmias from the endpoint.
REFERENCES:
- AVEIR DR FDA Approval.
- Knops, Reinoud E., et al. “A Dual-Chamber Leadless Pacemaker.” New England Journal of Medicine (2023). DOI: 10.1056/ NEJMoa2300080.
- AVEIR™ Leadless Pacemakers and Delivery Catheter IFU. ARTEN600284235.
- Sattar et al. Complications of leadless vs conventional (lead) artificial pacemakers - a retrospective review. Journal of community hospital internal medicine perspectives vol. 10,4 328-333. 2 Aug. 2020, doi:10.1080/20009666.2020.1786901.
- Reddy VY, Cantillon DJ, John IP . San Francisco, CA: 6 May 2016. A comparative study of acute and mid-term complications of leadless vs transvenous pacemakers. Late-Breaking Clinical Trials II. Presented at Heart Rhythm Society 2016; pp. 02–04. Abstract LBCT.

MAT-2404193 v1.0