Single and Dual Chamber Leadless Pacemaker Solutions, only from Abbott
.png)
MAT-2403640 v2.0 | Item approved for U.S. use only. ©2025 Abbott. All Rights Reserved.
AVEIR AR Atrial LP. AVEIR VR Ventricular LP. Individually, they are capable of AAI(R) or VVI(R). But together they can provide the world's first leadless DDD(R) system. Tailor therapy by implanting one or both devices based on your patient’s indications and needs.
Learn more about the benefits of this system that is redefining the landscape of cardiac pacing below.

AVEIR™ DR DUAL CHAMBER LP SYSTEM
To support dual chamber therapy, each implant communicates beat-to-beat with a paired, co-implanted device using i2i communication. This novel technology employs low energy, subthreshold pulses between implanted devices using the conductive nature of the body's blood pool and myocardial tissue. These high frequency pulses of data are delivered concurrently with each locally paced or sensed event, without impact on pacing or intrinsic sensing.

With sensing and pacing in both the right atrium and right ventricle, you now have AAI(R), VVI(R), DDD(R) and AAI(R)+VVI leadless configuration to consider. You can match your patients' pacing needs today and then upgrade over time as those needs change.
Treatment option 1: Start with the atrial device.
Treat sinus node dysfunction today.
Add a ventricular device for heart block later.

Enable i2i communication.

Activate dual chamber pacing therapy DDD(R) via i2i communication.

Treatment option 2: Start with the ventricular device.
Treat rare intermittent heart block today.

Add an atrial device for sick sinus syndrome later.

Enable i2i communication.

Activate dual chamber pacing therapy DDD(R) via i2i communication.

Treatment option 3: Start with a dual chamber DDD(R) system.
Start with a dual chamber system.

Turn off Beat-to-Beat communications to enable independent single chamber pacing for each device.

Treat SND patients today with AAI(R)+VVI pacing in the case of rare intermittent AV block.

This allows for the replacement of the atrial or ventricular device at end of service (EOS) without leaving hardware behind.
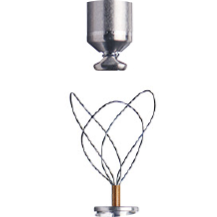
Specialty designed retrieval catheter supported by step-by-step protocol.
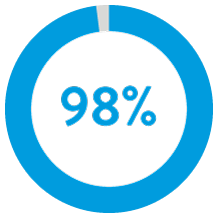
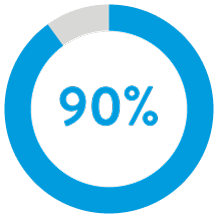
Through nine years regardless of implant duration, AVEIR™ VR Leadless Pacemaker (LP) predecessor device has a long-term retrieval success rate of 88%.3

Active fixation helix uses a screw-in mechanism to enable both implantation and long-term retrieval of the LP.
AVEIR VR and AVEIR AR LPs can measure P/R-waves, impedance and Current of Injury via commanded EGM, and an initial capture threshold before fixation by simply touching the electrode to the endocardial tissue.2 The LPs are engaged with a rotational motion in the endocardium, and mapping capability is designed to help reduce the number of repositioning attempts.2
References
MAT-2306873 v7.0
Stay Connected