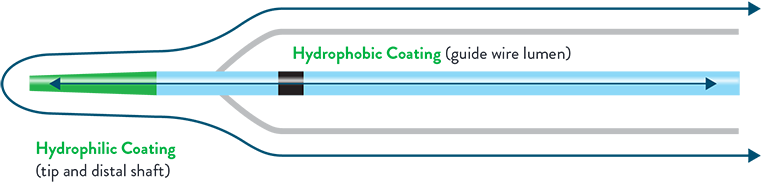
Strength and Non-Compliant Precision Without Compromising Deliverability
Diverse Balloon Portfolio
- The only non-compliant balloon platform available in all three guide wire compatible systems on the U.S. market
- Available in 200 cm shaft lengths for radial access and balloon working lengths from 20mm-240mm
Excellent Delivery
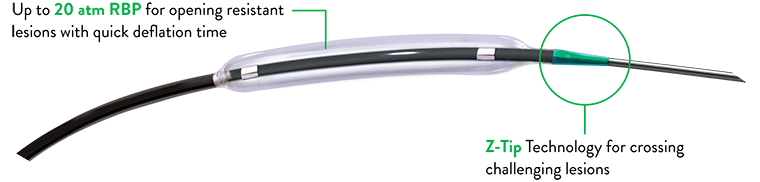
- Z-Tip technology offers exceptional entry and competitive crossing profile while proprietary dual balloon coating provides enhanced trackability
Safety and Accuracy
- Consistent uniform pressure distribution (minimizing dog-boning effect) during balloon dilation in all plaque morphologies
| Pressure (atm) | Balloon Diameter (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.51 | 2.0 | 2.5 | 3.0 | 3.52 | 4.0 | 5.0 | 6.0 | 7.03 | |
| 2 | 1.41 | 1.88 | 2.37 | 2.82 | 3.27 | 3.70 | 4.67 | 5.60 | 6.64 |
| 4 | 1.43 | 1.90 | 2.40 | 2.86 | 3.32 | 3.76 | 4.74 | 5.68 | 6.73 |
| 6 | 1.45 | 1.93 | 2.42 | 2.89 | 3.36 | 3.82 | 4.80 | 5.76 | 6.82 |
| 8 | 1.47 | 1.95 | 2.45 | 2.93 | 3.41 | 3.88 | 4.87 | 5.84 | 6.91 |
| 10 NOM* | 1.48 | 1.98 | 2.47 | 2.96 | 3.45 | 3.94 | 4.93 | 5.92 | 7.00 |
| 12 NOM* | 1.50 | 2.00 | 2.50 | 3.00 | 3.50 | 4.00 | 5.00 | 6.00 | 7.09 |
| 14 | 1.52 | 2.02 | 2.53 | 3.04 | 3.55 | 4.06 | 5.07 | 6.08 | 7.18 |
| 16 RBP** | 1.53 | 2.05 | 2.55 | 3.07 | 3.59 | 4.12 | 5.13 | 6.16 | 7.27 |
| 18 RBP** | 1.55 | 2.07 | 2.58 | 3.11 | 3.64 | 4.18 | 5.20 | 6.24 | 7.36 |
| 20 RBP** | 1.57 | 2.10 | 2.60 | 3.14 | 3.68 | 4.24 | 5.26 | 6.32 | 7.45 |
| 22 | 1.59 | 2.12 | 2.63 | 3.18 | 3.73 | 4.30 | 5.33 | 6.40 | — |
| 24 | 1.60 | 2.14 | 2.65 | 3.22 | 3.78 | 4.36 | 5.40 | — | — |
| 26 | 1.62 | 2.17 | 2.68 | 3.25 | 3.82 | 4.42 | — | — | — |
1. 1.5 mm configurations available only for .014 platform.
2. 3.5 configurations available only for .014 and .018 platforms.
3. 7.0 mm configurations available only for .018 and .035 platforms.
*Nominal Pressure. The nominal in-vitro device specifications do not take into account any lesion resistance.
**Rated Burst Pressure. Do not exceed RBP.
Manufactured by OrbusNeich Medical Group Holdings Limited or its affiliates. Distributed by Cardiovascular Systems, Inc. (CSI). CSI is a subsidiary of the Abbott Group of Companies.
JADE‡ and OrbusNeich are registered trademarks of OrbusNeich Medical Group Holdings Limited or its affiliates.
CAUTION: This OrbusNeich product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use at eifu.orbusneich.com for more detailed information on Indications, Contraindications, Warnings, Precautions, and Adverse Events
MAT-2404488 v2.0
IMPORTANT SAFETY INFORMATION
JADE‡ Rx, JADE‡ 014, JADE‡ 018, and JADE‡ 035
PTA Balloon Dilatation Catheters

INDICATIONS
The JADE‡ PTA Balloon Dilation Catheter is indicated for Percutaneous Transluminal Angioplasty in the peripheral vasculature, including iliac, femoral, iliofemoral, popliteal, infra-popliteal, and renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. This device is also indicated for post-dilation of balloon expandable and self-expanding stents in the peripheral vasculature.
CONTRAINDICATIONS
The use of the JADE‡ PTA Balloon Dilatation Catheter is contraindicated:
- For use in the coronary or neuro vasculature.
- Where there is the inability to cross the target lesion with a guidewire.
WARNINGS
When using this type of device, the following warnings should be observed:
- This device is intended for single use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of cross-contamination.
- When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Applying excessive force to the catheter can result in separation of the tip or balloon.
- To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.
- Balloon pressure should not exceed the rated burst pressure (RBP) indicated on the package. The rated burst pressure is based on the results of in vitro testing. At least 99.9 percent of the balloons, (with at least 95 percent confidence) will not burst at or below the rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization.
- To reduce the potential for air embolus into the vessel, use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon.
- For the rapid exchange catheters, do not re-straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft.
PRECAUTIONS
- The catheter system should be used only by physicians trained in percutaneous transluminal angioplasty.
- Use the catheter prior to the “Use By" date specified on the package.
- Prior to angioplasty, the catheter should be examined to verify functionality and ensure that its size and shape are suitable for the specific procedure for which it is to be used.
- Do not use oil-based contrast medium, organic solvents or alcohols; there is a possibility of catheter leak, damage or lubrication loss.
- The balloon deflation time has been established as 30 seconds (for 0.014” Rx) and 60 seconds (for 0.014”, 0.018”, and 0.035” OTW catheters) based on in vitro bench testing results.
- Use with caution for procedures involving calcified lesions or synthetic vascular grafts due to the abrasive nature of these lesions.
- Do not reinsert the PTA catheter into the coil dispenser after procedural use.
- Discard all disposable devices used during this procedure per local requirements for medical device waste disposal.
ADVERSE EFFECTS
Adverse effects due to the use of this product include, but are not limited to, the following:
- Acute or subacute thrombosis
- Acute vessel closure
- Allergic reaction to device, contrast medium, or medication
- Aneurysm
- Arrhythmias
- Arteriovenous fistula
- Death
- Dissection (perforation, rupture, or injury) of the vessel
- Hemorrhage or hematoma
- Hypertension
- Hypotension
- Infection
- Occlusion of the artery
- Restenosis of the dilated vessel
- Stroke, air embolism and embolization of fragmentation of thrombotic or atherosclerotic material
MAT-2400998 v2.0
