The HeartMate 3™ LVAD with Full MagLev™ Flow Technology has significantly advanced the field of LVAD therapy, setting the standard with innovation and outstanding clinical outcomes that make a meaningful difference in your patients’ lives.1
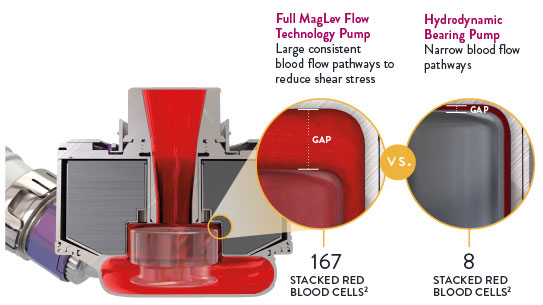
Full MagLev Flow Technology maintains gentle blood handling to minimize complications and hemocompatibility-related adverse events.1
The HeartMate 3 LVAD is used for advanced heart failure patients needing short- or long-term mechanical circulatory support.
Full MagLev Flow Technology maintains gentle blood handling to minimize complications and hemocompatibility-related adverse events.
Time may run out for patients put on the waiting list, to receive a heart transplantation. Their condition may require earlier intervention than a donor heart would become available. Some of these patients may benefit from a left ventricular assist device (LVAD), an implanted heart pump which can help the heart pump oxygen-rich blood throughout the body and potentially improve the symptoms of advanced heart failure.1
An LVAD is designed to restore blood flow and improve survival outcome, functional status, and quality of life in patients while minimizing complications such as stroke and thrombosis. Intended for a broad range of advanced heart failure patients, LVADs are an option for patients with NYHA Class IIIB or IV heart failure and can be used for both short-term and long-term heart support including:
You may find it reassuring to know that there are thousands of people around the world with LVADs living active, productive lives. They are spending time with friends and family and doing the things they love.5
HeartMate 3 LVADs set the standard in heart failure LVAD therapy through innovation, experience and outstanding outcomes.1 Our goal, like yours, is to keep patients with heart failure moving forward with the best possible quality of life.
MAT-2105166 v3.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0