Abbott technologies enable physicians to approach each patient with sophisticated insights from imaging and physiology assessments to identify the presence or absence of ischemia, and interventional therapies with proven safety that have become the industry’s gold standard.
As a global leader in cardiology, Abbott is dedicated to supporting healthcare professionals no matter how simple or complex their coronary artery disease (CAD) cases may be. We’re striving toward the same goal: offering optimal therapies, ensuring patient safety and improving health.
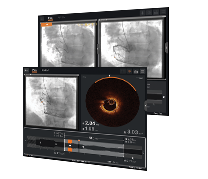
Optical coherence tomography (OCT) provides the intravascular imaging guidance that physicians increasingly turn to during PCI to guide decision-making. Abbott's OCT imaging system provides automated, accurate measurements to help guide stent selection, placement and deployment.1 OCT reduces ambiguities impacting vessel preparation, stent sizing and expansion to deliver optimal results.2 Discover the benefits of Abbott's OCT imaging today.
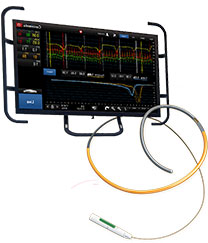
Physiological measurements such as fractional flow reserve (FFR) and resting full-cycle ratio (RFR) can be used to evaluate the functional significance of coronary stenosis.3,4 Beyond the visibility of angiography, index of microcirculatory resistance (IMR) and coronary flow reserve (CFR) can be used to diagnose coronary microvascular dysfunction.5,6 A comprehensive physiology assessment can improve PCI outcomes and patients' quality of life.5,7-8
The post-PCI decision about DAPT duration must be individualized for each patient. XIENCE™ Stents provide the only 28-day DAPT labeling option for patients at high bleeding risk.9 With the increasing number of complex cases, safety data on Abbott stents—for both short-term and long-term DAPT duration—can offer assurance to healthcare professionals focused on patient safety.10
MAT-2205024 v1.0

The Ultreon™ 1.0 Software is intended to be used only with compatible OPTIS™ Next Imaging Systems. The OPTIS™ Next Imaging System with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure. The OPTIS™ Next Imaging System is intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Contraindications: Use of the Ultreon™ 1.0 Software is contraindicated where introduction of any catheter would constitute a threat to patient safety.
Contraindications include:
Complications: The risks involved in vascular imaging include those associated with all catheterization procedures. The following complications may occur as a consequence of intravascular imaging and may necessitate additional medical treatment including surgical intervention.
Warnings:
Precautions:
MAT-2104193 v3.0

Indications:
The Ultreon™ 1.1 Software for Ultiri™ Measurement System is intended to be used only with compatible Ultiri™ Measurement Systems.
The Ultiri™ Measurement System is intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment, to determine if therapeutic intervention is indicated.
Contraindications:
There are no known contraindications for the Ultreon™ 1.1 Software for Ultiri™ Measurement System.
Warnings:
Precautions:
Potential Adverse Events:
Potential complications which may be encountered during all catheterization procedures include, but are not limited to:
MAT-2208957 v2.0

Indications: CoroFlow‡ is indicated to provide hemodynamic information for use in the diagnosis of patients with cardiovascular diseases.
CoroFlow‡ is intended for use in catheterization and related cardiovascular specialty laboratories to compute and display various physiological parameters based on the output from one or more measuring devices.
Contraindications: The system has no patient alarm functions. Do not use for cardiac/vital signs monitoring.
Warnings:
Precautions:
MAT-2007904 v3.0

Indications
Applies to XIENCE Skypoint™ Stent Systems:
Applies to XIENCE Sierra™ and XIENCE Alpine™ Stent Systems:
Contraindications
The XIENCE Skypoint™, XIENCE Sierra™ and XIENCE Alpine™ Stent Systems are contraindicated for use in:
Warnings
Failure to abide by the warnings in this labeling might result in damage to the device coating, which may necessitate intervention or result in serious adverse events.
Precautions
Potential Adverse Events
Adverse events that may be associated with PCI treatment procedures and the use of a stent in native coronary arteries include, but are not limited to, the following:
The risks described below include the anticipated adverse events relevant for the cardiac population referenced in the contraindications, warnings and precaution sections of the everolimus labels / SmPCs and / or observed at incidences ≥ 10% in clinical trials with oral everolimus for different indications. Please refer to the drug SmPCs and labels for more detailed information and less frequent adverse events.
There may be other potential adverse events that are unforeseen at this time.
MAT-2100879 v7.0
Stay Connected