The Supera™ Peripheral Stent is indicated for the superficial femoral artery (SFA) and the proximal popliteal artery. Engineered by a unique interwoven wire technology, this nitinol stent offers physicians unmatched clinical outcomes5-16 across varied lesion complexities and lengths.1-4
To learn more about Supera™ Stent, simply request a free demonstration and your local Abbott representative will be in touch shortly.
The Supera™ Stent is known for the excellence of its clinical outcomes during percutaneous transluminal angioplasty (PTA) procedures, since this peripheral stent has been studied in more than 2,000 patients and 17 studies worldwide.3,5-16
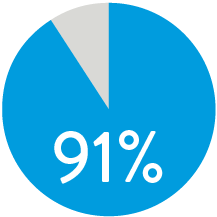
PATENCY (K-M) AT 1 YEAR5
When nominally deployed*
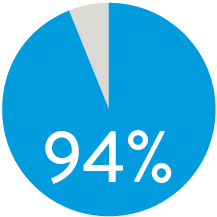
FREEDOM FROM TLR AT 3 YEARS5
When nominally deployed*
*Nominal deployment is defined as the stent length upon deployment being within +/- 10% of the labeled stent length. This data is from a non-powered post-hoc analysis.
Supera™ Stent demonstrated excellent 1 yr patency and 3 yr freedom from TLR in the SUPERB trial.5
Demonstrated unmatched clinical outcomes in simple lesions across US pivotal stent trials5-20
Exhibits consistent 1-year primary patency results regardless of lesion length17-25
Reveals strong clinical outcomes in severely calcified lesions at year 3 years5
* Study reported a majority with Trans-Atlantic Inter-Society Consensus Document (TASC) A & B lesions and/or Rutherford class 2 or 3 lesions
Unlike any other stent design platform, the Supera™ Stent is uniquely designed to keep vessels open with its distinct platform, created by interwoven individual, flexible nitinol wires
4x greater strength for compression resistance—so it can maintain a round, open lumen, which can be especially beneficial in calcified lesions
With 1:1 stent to vessel sizing, low chronic outward force results in minimal vessel injury28
Unparalleled flexibility,27 which mimics the natural structure and movement of the anatomy29-31
Zero stent fractures reported at 1 year in over 2,000 patients across 17 studies3,5-20
MAT-2109821 v2.0

Indications
The Supera™ Peripheral Stent System is indicated to improve luminal diameter in the treatment of patients with symptomatic de novo or restenotic native lesions or occlusions of the superficial femoral artery (SFA) and / or proximal popliteal artery with reference vessel diameters of 4.0 to 7.5 mm, and lesion lengths up to 140 mm.
Contraindications
The Supera™ Peripheral Stent System is contraindicated in:
Warnings
Precautions
The Supera™ Peripheral Stent System should only be used by physicians and medical personnel trained in vascular interventional techniques and trained on the use of this device.
Magnetic Resonance Imaging (MRI) Safety Information
Nonclinical testing has demonstrated that the Supera™ stent, in single and in overlapped configurations up to 250 mm in length, is MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions:
Under the scan conditions defined above, the Supera™ stent is expected to produce a maximum temperature rise of 7.6 °C after 15 minutes of continuous scanning.
In nonclinical testing, the image artifact caused by the device extends approximately 2 cm from the Supera™ stent when imaged with a gradient echo or spin echo sequence and a 3T MRI system.
Potential Adverse Events
Potential adverse events include, but are not limited to:
MAT-2103597 v3.0
Stay Connected