The Supera™ Stent has been studied in over 2,000 patients worldwide in the SUPERB trial and 16 retrospective studies. Notably, in all of the 17 studies, the Supera™ Peripheral Stent showed durable results with zero fractures at 1 year.1,3-6,9-12,14-21
At 1 year the Supera™ Stent demonstrated primary patency of 91% when nominally* deployed. At 3 years, freedom from targeted lesion revascularization (TLR) was 94% when nominally* deployed.1
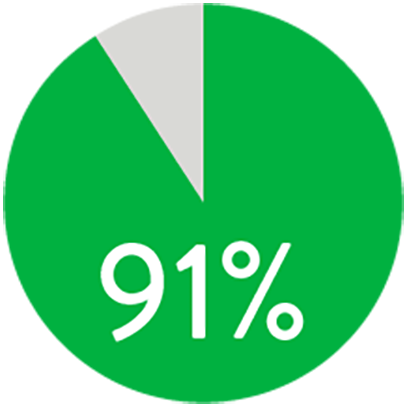
PATENCY (K-M) AT 1 YEAR
When nominally deployed*
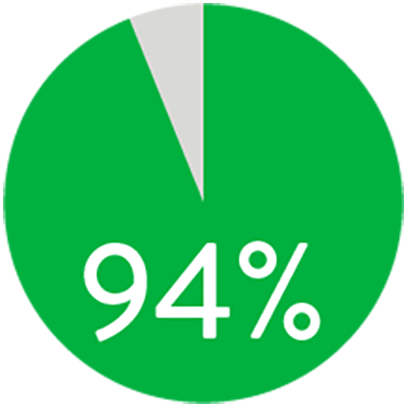
FREEDOM FROM TLR AT 3 YEARS
When nominally deployed*
*Nominal deployment is defined as the stent length upon deployment being within +/- 10% of the labeled stent length. This data is from a non-powered post-hoc analysis. K-M=Kaplan Meier.
With some peripheral stents, increasing lesion lengths can lead to decreasing patency rates.20 The Supera™ Stent stands apart for its consistently high patency rates in lesions spanning lengths from 5.3 cm up to 28.0 cm*.
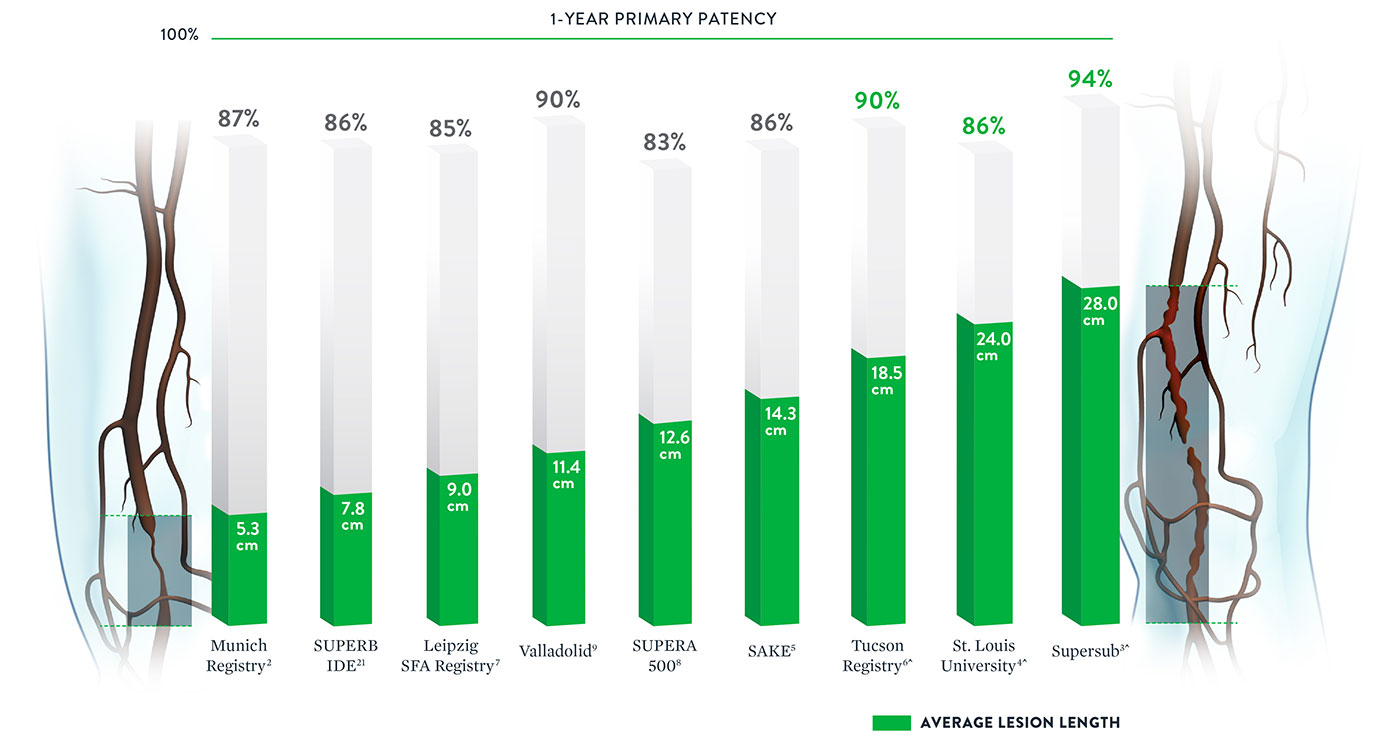
*Published data was included if lesion length and patency were both available. ^Multiple stents used for longer length.
Note: Results from different clinical trials are not directly comparable. Information provided for educational purposes only.
Whether treating simple (TASC A&B) or complex (TASC C&D) lesions, the Supera™ Stent is associated with impressive, consistent patency performance data.2-4,21
| Simple | |||
|---|---|---|---|
 | Trial/Study | Munich Registry2 | SUPERB21 |
| Lesion Length | 5.3cm | 7.8cm | |
| TASC A&B Lesions | 100% | 94% | |
| 1-Yr Patency | 86.7% | 90.5% | |
| Sites | Single Center | Multicenter (46 sites) | |
| # Patients | 70 | 264 | |
TASC: Trans-Atlantic Inter-Society Consensus
| Complex | |||
|---|---|---|---|
 | Trial/Study | St. Louis4 | SuperSub3 |
| Lesion Length | 5.3cm | 7.8cm | |
| TASC C&D Lesions | 78% | 100% | |
| CTOs | Unknown | 100% | |
| 1-Yr Patency | 85.6% | 94.1% | |
| Sites | Single Center | Single Center | |
| # Patients | 48 | 34 | |
TASC: Trans-Atlantic Inter-Society Consensus
MAT-2109822 v3.0

Indications
The Supera™ Peripheral Stent System is indicated to improve luminal diameter in the treatment of patients with symptomatic de novo or restenotic native lesions or occlusions of the superficial femoral artery (SFA) and / or proximal popliteal artery with reference vessel diameters of 4.0 to 7.5 mm, and lesion lengths up to 140 mm.
Contraindications
The Supera™ Peripheral Stent System is contraindicated in:
Warnings
Precautions
The Supera™ Peripheral Stent System should only be used by physicians and medical personnel trained in vascular interventional techniques and trained on the use of this device.
Magnetic Resonance Imaging (MRI) Safety Information
Nonclinical testing has demonstrated that the Supera™ stent, in single and in overlapped configurations up to 250 mm in length, is MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions:
Under the scan conditions defined above, the Supera™ stent is expected to produce a maximum temperature rise of 7.6 °C after 15 minutes of continuous scanning.
In nonclinical testing, the image artifact caused by the device extends approximately 2 cm from the Supera™ stent when imaged with a gradient echo or spin echo sequence and a 3T MRI system.
Potential Adverse Events
Potential adverse events include, but are not limited to:
MAT-2103597 v3.0
Stay Connected