The Supera™ Stent’s unique design results in a platform with features unmatched among standard nitinol peripheral stents:
The interwoven nitinol design provides high structural integrity and kink resistance, producing the features that set the Supera™ Stent apart.
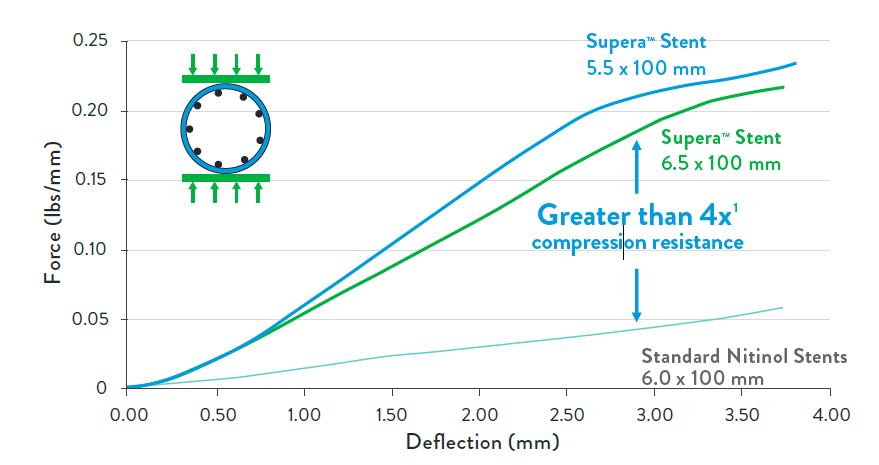
The Supera™ Stent exhibits more than 4 times the compression resistance of all other self-expanding nitinol stents, which are laser cut from an inflexible nitinol tube.2 Benchtop testing with the 5 pounds of force results in compression of a standard laser-cut nitinol stent (below left). When the same force is applied to the Supera™ Stent, it retains its circular form (below right).
In the clinical setting, those results are illustrated—by being deployed in the same patient and same vessel—via the angiogram and intravacular ultrasound (IVUS) images below. In the IVUS images, note the compression of the standard nitinol stent, while the Supera™ Stent was able to retain its circular shape. Such performance is particularly important in heavily calcified lesions.
Optimizes luminal gain and maintains circular geometry
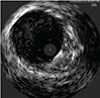
SUPERA™ STENT21
Same artery shown, with a Supera™ Stent distal to the SNS
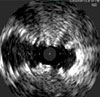
SNS21

Source: Angiogram and IVUS images courtesy of Dr. Andrej Schmidt.
While laser-cut nitinol stents are designed and required to be oversized for deployment, the Supera™ Stent is unique in that it is sized 1:1 with the vessel diameter. So as seen in the image below, the laser-cut stents inherently exert force on the vessel wall throughout the lifetime of the stent, which in turn can result in endovascular injury over the long term. The lower chronic outward force (COF) with the Supera™ Stent results in fewer vessel injuries.1,5
 | Pushes against the vessel to open, through lifetime of SNS |  |  |
| Stent Oversizing | High COF6 | Vessel Injury4 | |
|---|---|---|---|
| Chronic outward force (COF) is exerted on vessel by self-expanding stents due to inherent oversizing | |||
 | 1:1 sizing allows to scaffold the vessel to maintain open |  |  |
| Sizing 1:1 | Low COF6 | Minimal Vessel Injury4 | |
|---|---|---|---|
| Supera™ Stent is sized 1:1 with the prepared vessel and therefore has minimal chronic outward force | |||
All other self-expanding nitinol stents are fashioned from a rigid, inflexible nitinol tube. As shown in the photo below, such laser-cut stents are less likely to conform to a dynamic vascular environment and can potentially kink and fracture in tortuous vessels (left).


With the Supera™ Stent, however, individual flexible nitinol wires are interwoven for unparalleled flexibility,5 excellent kink resistance, and the ability to mimic the natural movement of the anatomy1,7,8 (right). Given the twisting and compression characteristics of the superficial femoral artery and proximal popliteal, this stent is an effective choice.
Consequently, data on over 2,000 patients published in 17 studies have shown that at 1 year the Supera™ Stent has zero fractures.4,9-24
in 1 year across all studies4,9-24

MAT-2109824 v2.0

Indications
The Supera™ Peripheral Stent System is indicated to improve luminal diameter in the treatment of patients with symptomatic de novo or restenotic native lesions or occlusions of the superficial femoral artery (SFA) and / or proximal popliteal artery with reference vessel diameters of 4.0 to 7.5 mm, and lesion lengths up to 140 mm.
Contraindications
The Supera™ Peripheral Stent System is contraindicated in:
Warnings
Precautions
The Supera™ Peripheral Stent System should only be used by physicians and medical personnel trained in vascular interventional techniques and trained on the use of this device.
Magnetic Resonance Imaging (MRI) Safety Information
Nonclinical testing has demonstrated that the Supera™ stent, in single and in overlapped configurations up to 250 mm in length, is MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions:
Under the scan conditions defined above, the Supera™ stent is expected to produce a maximum temperature rise of 7.6 °C after 15 minutes of continuous scanning.
In nonclinical testing, the image artifact caused by the device extends approximately 2 cm from the Supera™ stent when imaged with a gradient echo or spin echo sequence and a 3T MRI system.
Potential Adverse Events
Potential adverse events include, but are not limited to:
MAT-2103597 v3.0
Stay Connected